HOW-TO COVID-19: from device sanitisation to accessories to wear for maximum safety
Where ASA has always considered the control, cleaning and hygiene of the devices a must for their use, the Covid-19 emergency has further emphasised their importance, together with the sanitisation of the rooms where the treatment takes place and the clothing worn by the physiotherapist.
In order to keep the possible spread of the virus under control, in addition to social distancing, it is strongly recommended to adopt the instructions which are already provided when supplying the materials under "Cleaning and Disinfection".
Few, but clear rules to be applied, functional also to make the patient safer, by keeping the psychological gap concerning the fear of contact under control: regularly clean and disinfect the device and the applicators connected to it by using a soft cloth moistened with non-flammable and alcohol-free detergent - Benzalkonium chloride and ESO S80 (a compound of benzalkonium chloride and chlorhexidine digluconate) are already being used in Asa, while isopropyl alcohol is potentially harmful when used on plastic components, but not on optical ones, editor's note - and then dry with a clean cloth.
Compatibility of laser safety eyewear against common cleaning/disinfection substances
After undergoing several in-house experimental tests, our supplier defined the below list of recommended products/substances that might be updated in the future.
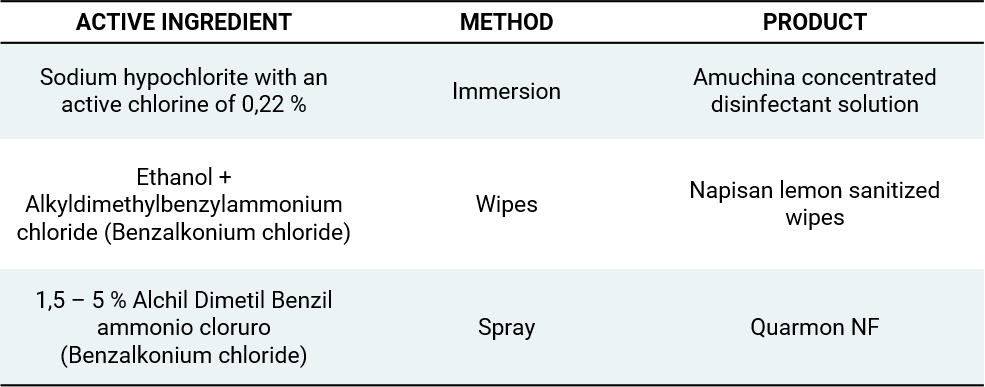
Proceed with a complete drying phase to avoid any possible build-up of the liquid in the geometries of the spectacle. It is crucial to always check the conformity and the integrity of the eyewear according to the information listed in the User Instruction that is included with every product, before and after the exposure of the substance, and before the use.
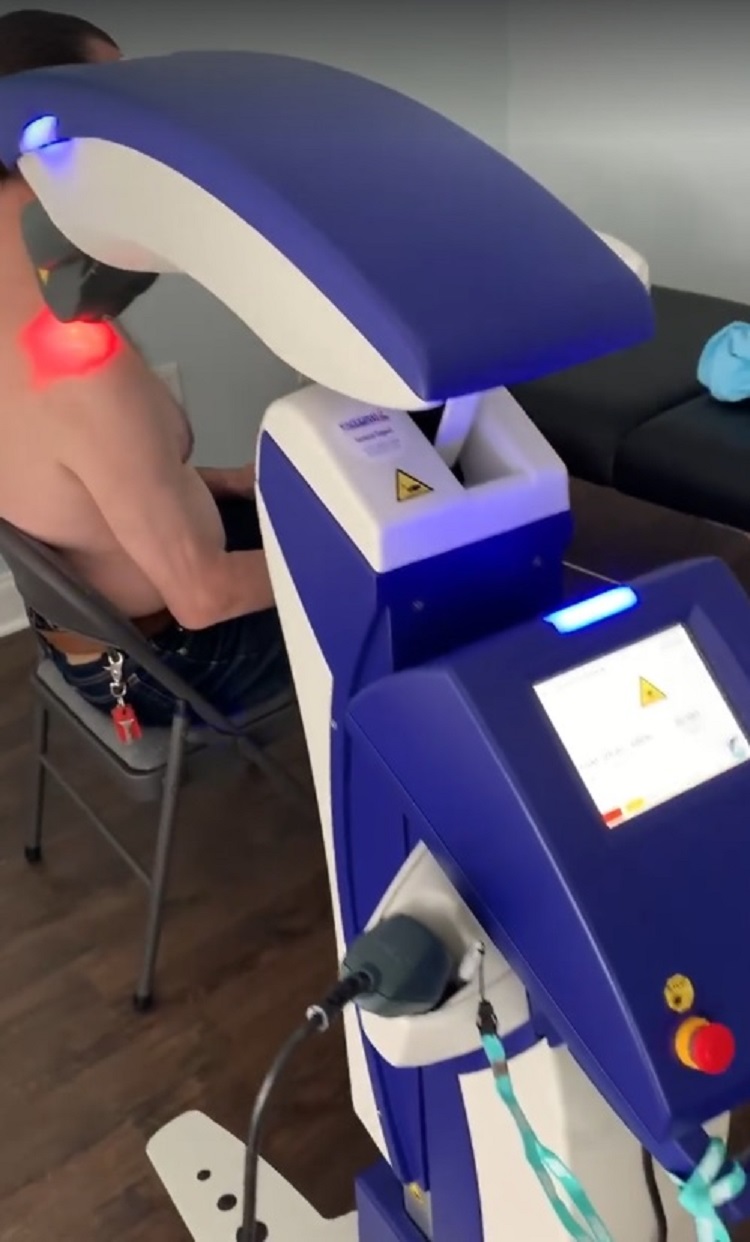
Interchangeable light guides
Although it is necessary to be very careful not to contaminate the optical unit, in order to guarantee a deep hygiene of the devices and at the same time to ensure their ready use, ASA offers a series of ad hoc accessories. An example is given by the interchangeable light guides for Mphi 75 supplied with a handbook explaining their maintenance: starting from the immersion of the light guide in a solution of soap and water (alternatively you can use a disinfectant wipe), use, if necessary, of a brush or soft cloth to manually remove any contamination, then rinsing all the components with running hot water and visually checking for any residues. Finish by drying the various elements with a clean cloth.
"As is also indicated in the user manual – the ASA Service Department reminds us - the light guides must be sterilised at first use and after each use in a steam autoclave with standard sterilisation programs at 134° -138°C, with a pressure of 2.1 bar, for 3.5-5 minutes".

The how-to linked to caring for the instrument goes hand in hand with that concerning the professional's clothing.
"Without forgoing an inclusive and welcoming environment, it is appropriate to review procedures and flows within your centre. In addition to letting in only a fixed number of patients, to reviewing the attendance of the collaborators, to using all the necessary PPEs (mask, visor, gown, shoe covers, gloves,...), it is essential to sanitise environments, reusable instruments and PPEs at the end of each session".
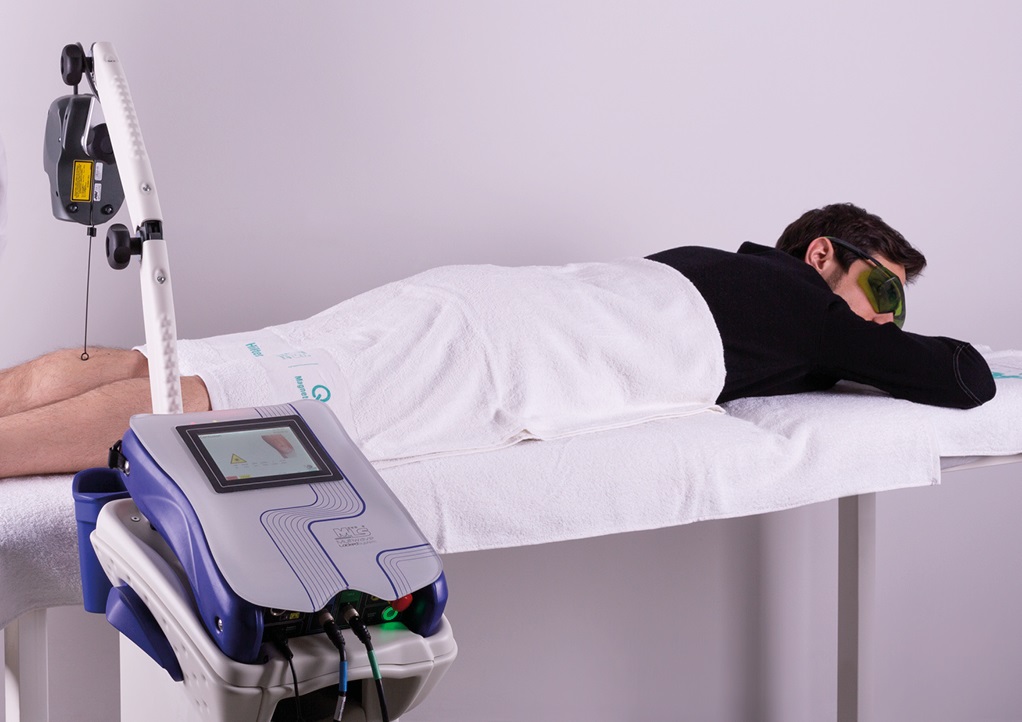
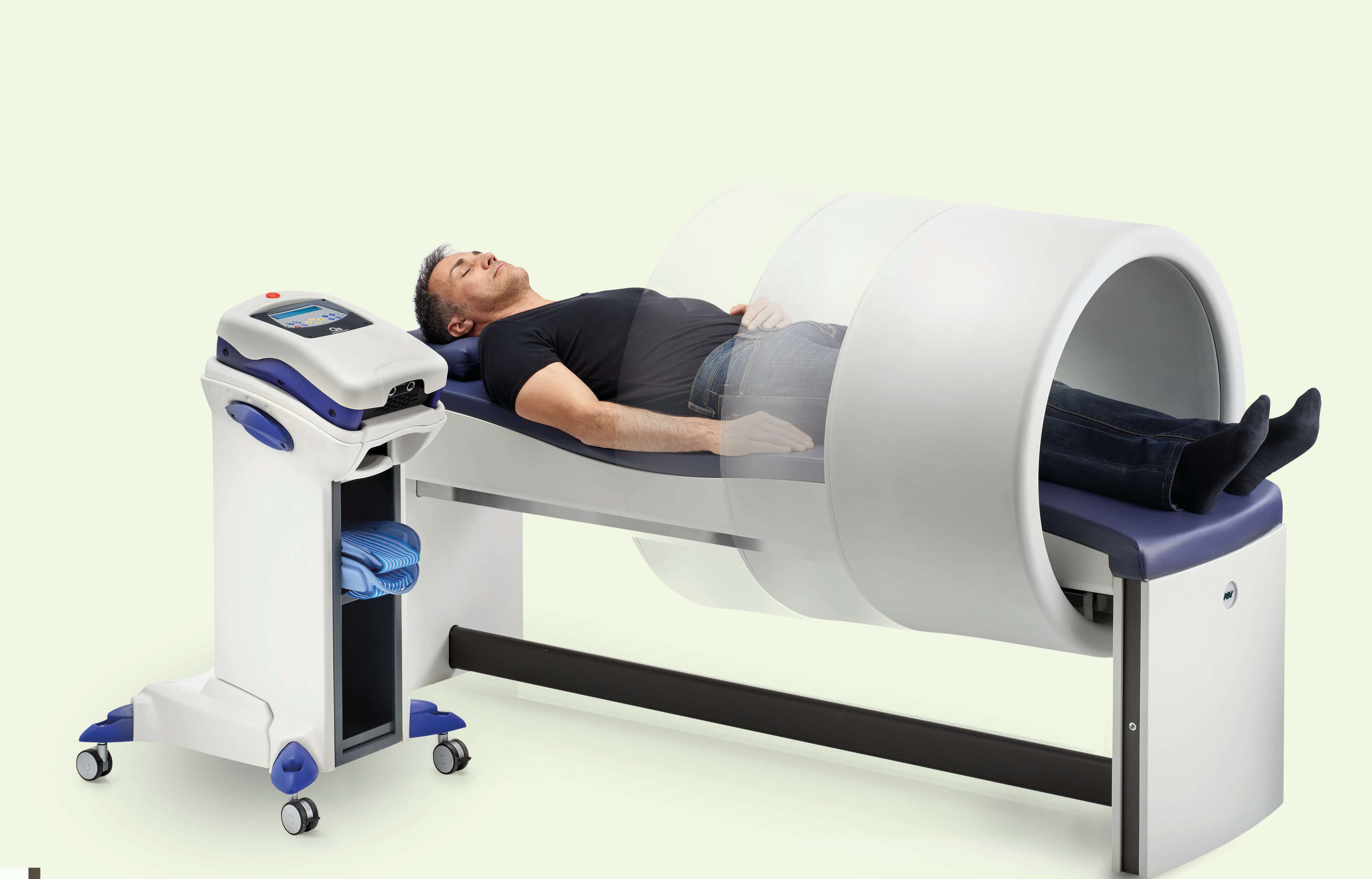
In the name of maximum safety, it should also be remembered that ASA products such as, for example, M6 and Mphi5 for MLS® Laser Therapy and Easy Qs and PMT Qs for Magnetotherapy, are operator-independent devices: the physiotherapist only has to set the parameters and supervise how the session is going as this occurs in total autonomy without any proximity or contact.