Social Distancing and Physiotherapy: no contact thanks to operator-independent devices
In the Covid-19 era there is a new term which has become a part of the vocabulary of us all and which has led to a substantial revision of our lifestyle and work: "social distancing".
Keeping at least 1 metre between one person and the other in order to limit the potential spread of the virus is not just a suggestion or a good practice, but an actual diktat that has imposed new approach methods to all those professions in which human contact is an "instrument".
Physiotherapists know this all too well, as they need to touch the body and be close to their patient. An operational gap added to a psychological component: the patient's fear of being contaminated by the approach of another person or by contact with the instruments.
In order to overcome this barrier and to reassure the patient, in addition to applying the ABCs in terms of cleaning and sanitising, being able to guarantee that the individual patient only undergoes treatments which do not involve intensive physical contact and which only require operator supervision is a real advantage.
ASA devices and distancing
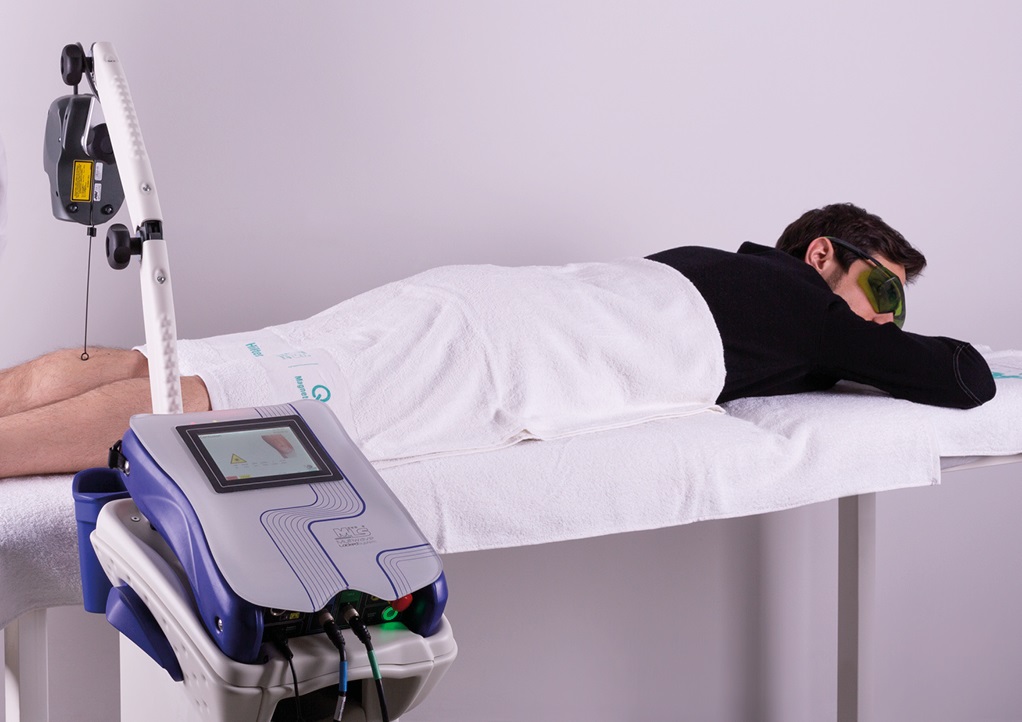
ASA physiotherapy and rehabilitation devices go in this direction as is confirmed, for example, by M6, the MLS® Laser Therapy device. The movement of its robotic head is in fact managed through an advanced user interface which only requires the therapist to supervise the treatment, carried out safely, effectively and autonomously. The possible risk of contamination is also kept under control as there is no contact between the robotic head and the patient. A similar principle also qualifies Mphi5 thanks to the Charlie multidiode applicator: the physiotherapist only has to set the parameters and check the progress of the application which takes place totally safely and without any proximity.

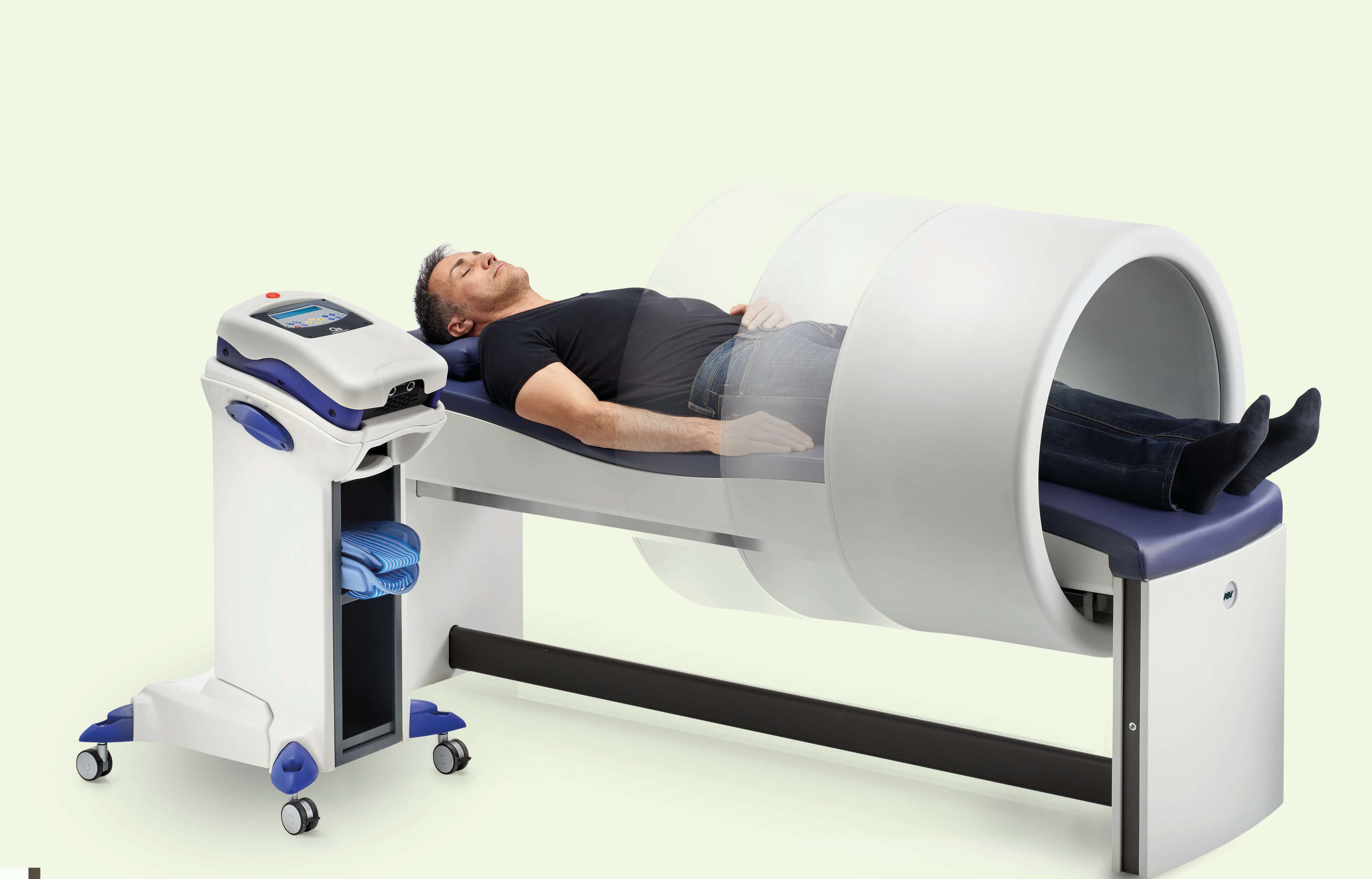
Lastly, the devices for Magnetotherapy Easy Qs and PMT Qs, for which the safety factor is further enhanced by the possibility for the patient to be treated whilst dressed, are also "operator-independent".
"Social distancing" can therefore not be a limit if therapeutic solutions entrusted to devices designed to respect and ensure this are used. All this without compromising the effectiveness of the session, always having at heart the primary need of those undergoing it: to recover their well-being and the best physical condition.