Video Conference Worldwide: a constant support to specialists and distributors
Supporting our partners is a diktat for ASA which, even during these "lock down" times in many Nations, has implemented a calendar of online meetings with its partners spread all over the world.
Objective: to keep our finger on the pulse of each country’s condition and, at the same time, to offer the opportunity to exchange views and to refresh the therapies.
From mid-February, the interactions between corporate headquarters in Italy with business partners around the world, from Australia to Ecuador, have never stopped.
Giacomo Granozio, ASA’s Area Manager for the Far East confirms this:
“The emergency we are experiencing has spurred us to further exploit various remote communication methods which ensure continuity and proximity to our business partners. For this purpose, we organise daily video-meetings in order to take stock of ongoing activities, to have a precise picture of the countries in terms of the incidence of the virus and of the consequent government decisions, as well as to provide practical indications on how to present Hilterapia®, MLS Laser Therapy® and Magnetotherapy in the most complete and exhaustive way. In some cases we focus our attention on the devices, providing details for best understanding their pluses, as occurred for example with our Indian and Czech distributors: in fact, we implemented a specific focus on MiS to the various vendors divided into groups in order to facilitate the flow of questions/answers".
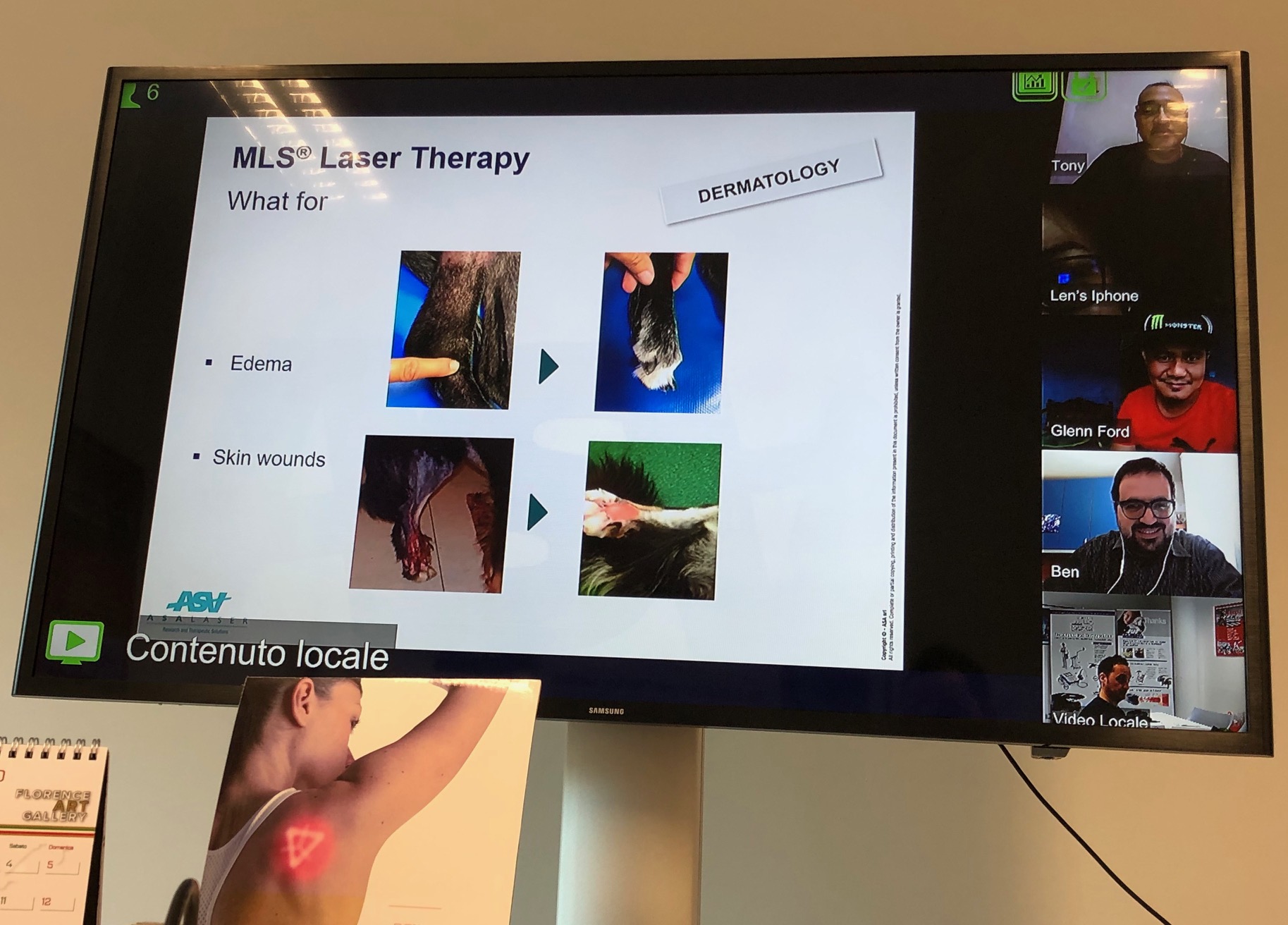
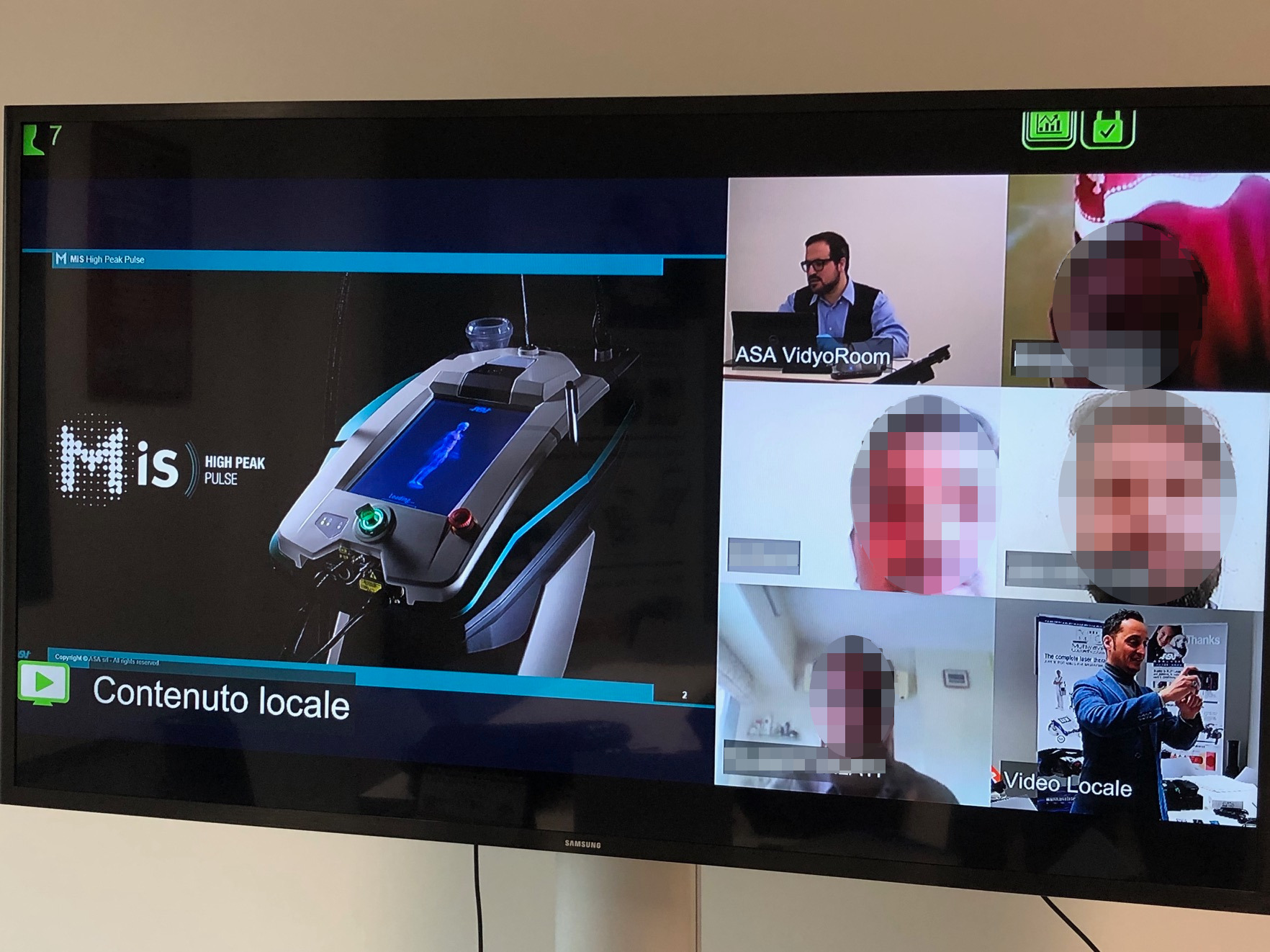
The remote intervention, however, also concerns aspects of a more formative nature.
“With our partner in the Philippines - explains Beniamino Caruso, ASA’s Area Manager – we devoted an in-depth analysis to the questions most frequently asked during demos dedicated to MLS® Laser Therapy, in order to assist them in creating performing and incisive presentations. With the Australian distributor, on the other hand, we addressed the use of MiS in peripheral neuropathies, reaffirming the importance of the research carried out which confirms its effectiveness”.
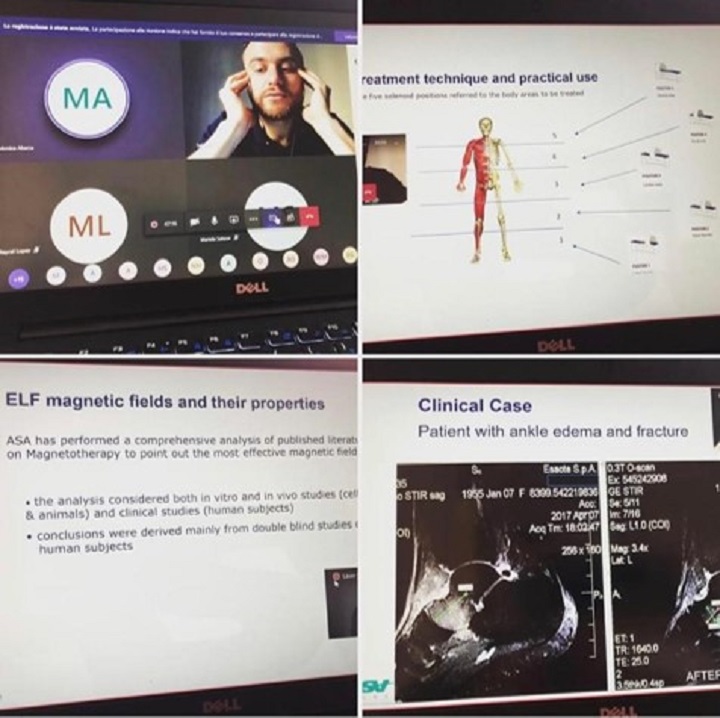
The devices’ pluses, the insights on the effective use of the therapies in the treatment of specific diseases and the presentation of targeted clinical cases were the focus of the virtual training sessions also held in Central and South America, with Costa Rica and Ecuador in the forefront.
“Video-conferences - explains Karla Salazar, ASA’s Area Manager - are a valid solution to maintain constant contact with both loyal customers and new interlocutors. A proactive attitude is a winner during times like these which require both resilience and flexibility by everyone. Putting our own skills and those of ASA trainers at the service of your business partner is essential to make the difference, yet again”.
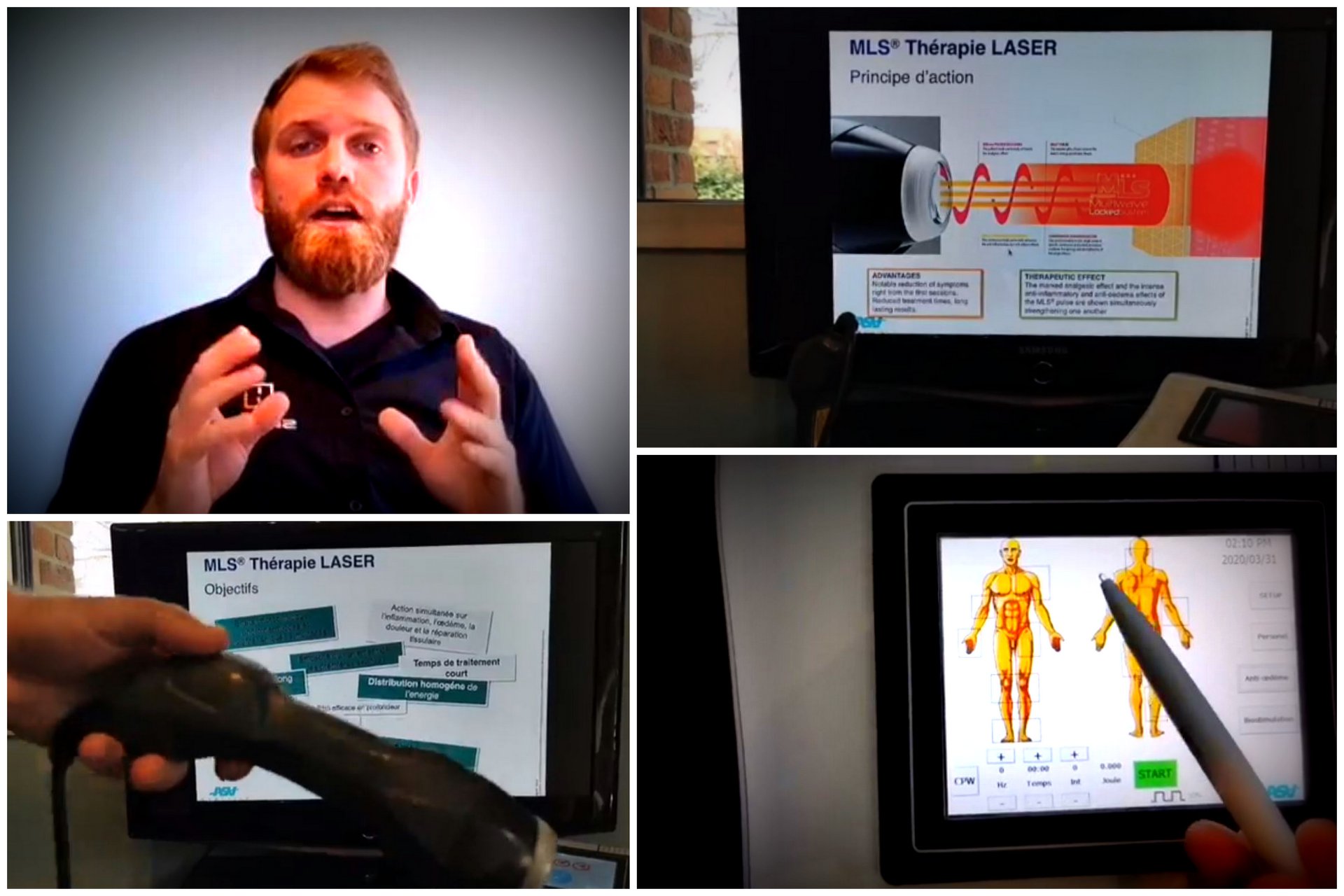
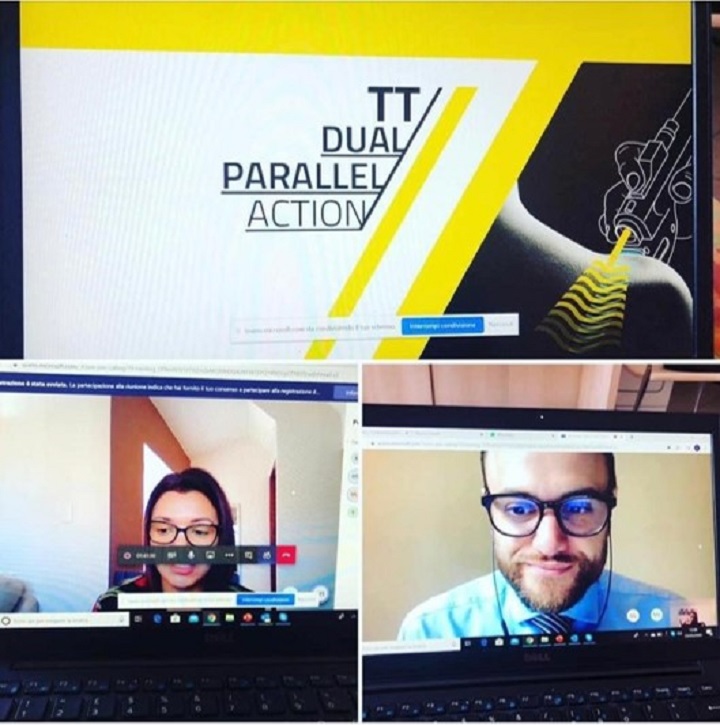
A similar approach was also adopted with the European contacts, with France, Spain and Sweden as leaders.
“Online training dedicated in particular to MLS® in the human and veterinary field - explains Roberto Terruzzi, ASA’s Senior Export Manager - marked the training calendar during this period, allowing participants to resume important concepts in terms of therapy usefulness and of its benefits as well as to ask new questions resulting from the experience gained over the past few months. We are engaged in organising remote meetings on a daily basis with our Swedish, German and Danish contacts and, to follow, with the Slovenian, Cypriot and Polish ones in the same way”.










