ASA expands MLS® Laser Therapy in South America: training, innovation and new business opportunities
ASA continues its expansion in Latin America, strengthening the presence of MLS® Laser Therapy among doctors, physiotherapists and specialists in orthopaedics and rehabilitation. With a series of events, training courses and meetings with the main sector professionals, ASA has consolidated its reference role for laser therapy.
Colombia: Innovation in orthopaedic rehabilitation
In collaboration with its partner Neomedic, ASA organised an event in Barranquilla with over 50 specialist doctors and physiotherapists. During the meeting, Giacomo Granozio, ASA Education Program Manager, illustrated the benefits of MLS® Laser Therapy through scientific studies and clinical cases.A striking case was that of the Clinica La Misericordia, which treated 50 patients with the M8 robotic device, obtaining significant clinical results. In addition, the proposed business model – which integrates MLS®, Hiro TT MLS®, Hiro TT and Qs Magnetotherapy – is creating new opportunities for the orthopaedic sector.




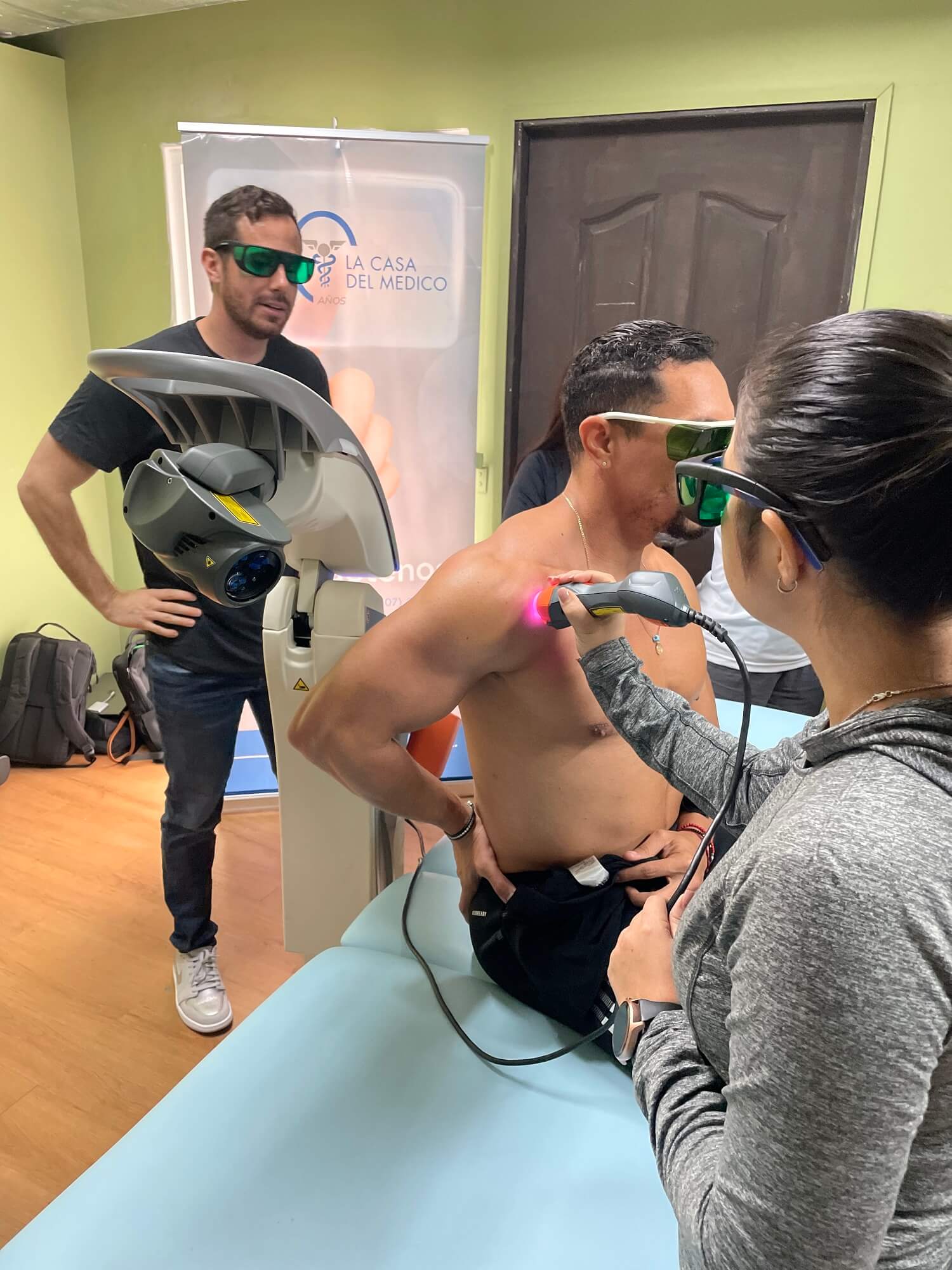
Panama: Laser therapy and Tecar therapy in synergy
In Panama, ASA collaborated with its partner Casa del Medico to demonstrate the effectiveness of the integration between laser therapy and Tecar therapy. A training course with over 30 specialists, including the president of the Panamanian Association of Physiotherapy and Kinesiology (APAFIK), highlighted how the two technologies are complementary for the effective treatment of musculoskeletal disorders.
Given its success, the course will be repeated in other cities to expand knowledge of the MLS® technology among Panamanian physiotherapists.



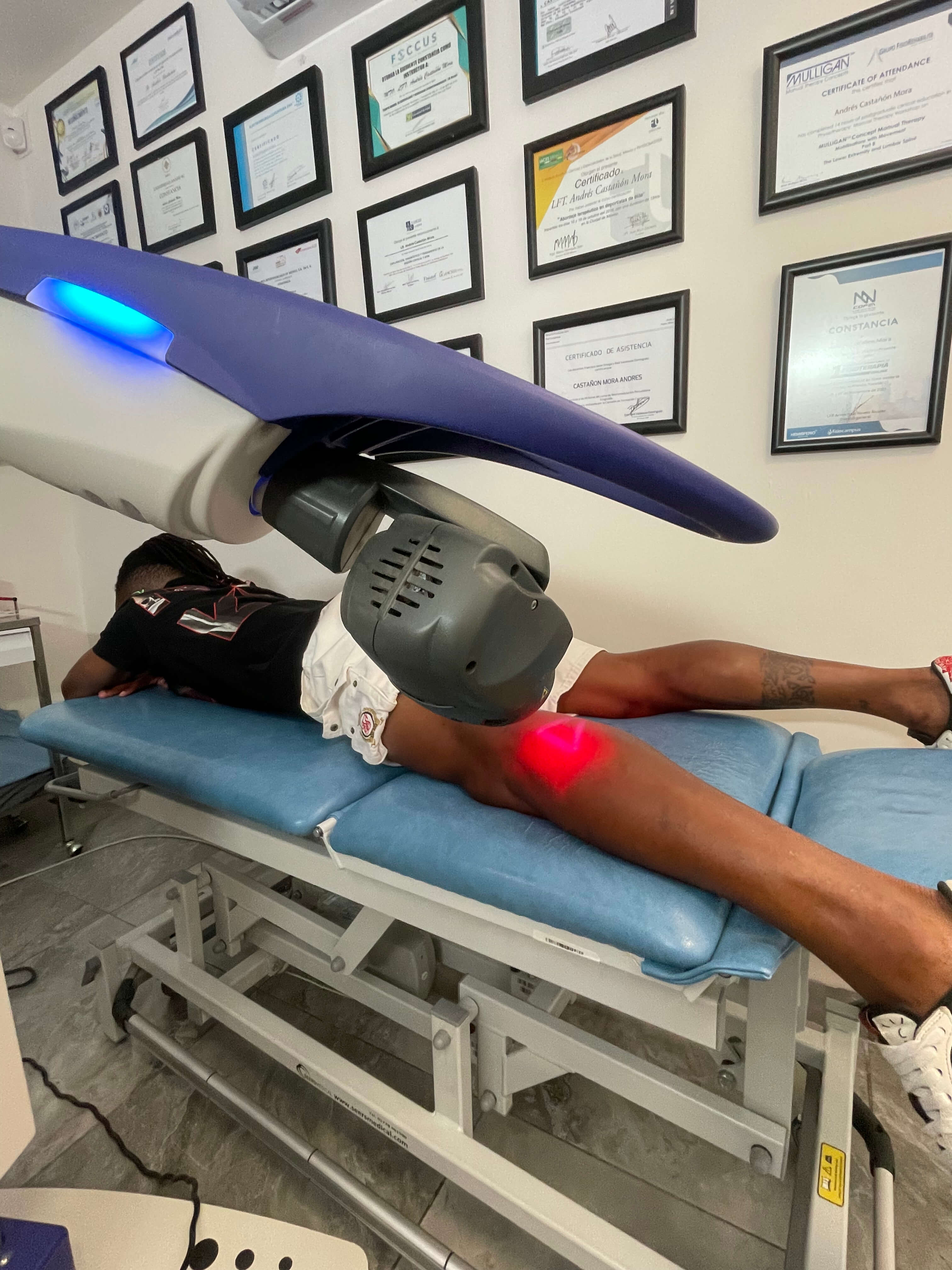
Mexico: training and clinical successes
The activity in Mexico, with the partner Equipos Interferenciales, included clinical demonstrations, meetings with Universities and specialists. The most important results included:
- At the Universidad Tecnológica Emiliano Zapata, laser therapy has been included in the study curriculum, marking a fundamental milestone for training future physiotherapists.
- In San Luis Potosí, numerous statements from professional athletes, including footballers and Olympic swimmers, to demonstrate the benefits of laser therapy in sports.




ASA strengthens MLS® Laser Therapy in international markets
Thanks to this intense activity, ASA continues to promote MLS® Laser Therapy and Hilterapia® as innovative tools for the treatment of orthopaedic and musculoskeletal disorders: the commitment to training doctors and physiotherapists and the dissemination of successful clinical cases are the basis of the growth of the ASA technologies in Latin American markets.










