Diabetes and Peripheral Neuropathies: 70% reduction in pain with MiS laser
Neuropathic pain is a debilitating condition caused by lesions or diseases that affect the somatosensory nervous system, often resistant to traditional treatments such as anti-inflammatories or opioids. Diabetes, a major cause of neuropathic pain, can lead to diabetic peripheral neuropathy, characterised by pain, numbness and burning sensations, with a significant impact on quality of life.
The study on a patient with type 2 diabetes mellitus
This clinical case highlights the effectiveness of using the MiS laser device, belonging to the patented MLS® family and designed to treat peripheral neuropathies, on a 67-year-old woman who was diagnosed with type II diabetes mellitus (adult-onset diabetes) in 2008, for which she has been undergoing insulin therapy since 2014.
The patient had hip osteoarthritis (coxarthrosis) on both sides and knee osteoarthritis (gonarthrosis) on the right side. In 2019 she began to complain of pain, numbness and a burning feeling in her legs and feet, symptoms that initially appeared on the right side and afterwards, in 2020, also on the left side.
In October 2022, she was diagnosed with distal sensorimotor polyneuropathy in the lower limbs. The electromyography (EMG) showed axonal degeneration and partial segmental demyelination, more marked in the sensory nerve fibres. The patient also reported a loss of skin sensitivity in the lateral part of the left thigh.
The turning point: treatment with MLS® Laser Therapy
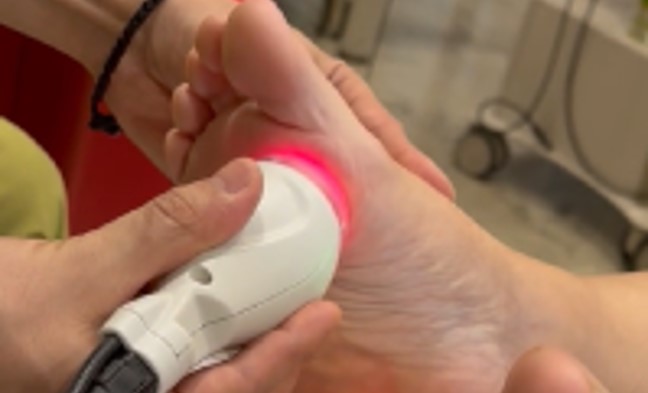
Despite conventional treatments, her symptoms persisted until, in November 2023, she underwent MLS® Laser Therapy. The therapy included six sessions over two weeks: each session targeted the feet and lower limbs with specific parameters based on EU MDR approved clinical guidelines.
Initially with scan treatment on the dorsal and plantar area of both feet and subsequently in fixed-point mode on both lower limbs.
The patient reported significant relief from symptoms:
- the disappearance of the burning and numbness in the left leg after four sessions and in the right leg after five.
- The improvement in skin sensitivity.
An assessment using the Neuropathic Pain Symptom Inventory (NPSI) showed a 70% reduction in pain, going from 67/100 to 20/100. The benefits were confirmed at one-month follow-up.
MiS laser and treatment of peripheral neuropathies
From a biological point of view, treatment with MiS promotes the recovery of damaged nerve fibres in the lesion area, as confirmed by histological and immunohistochemical analyses. In particular, reorganisation of the sheath is important for both nerve trophism and conduction. This case highlights the potential of MiS as an effective and non-invasive alternative for patients who are refractory to traditional treatments, paving the way for better results in the management of neuropathic pain.
Successful Management of Diabetic Peripheral Neuropathic Pain Using MiS MLS® Laser Therapy: A Case Report
I. Todorov
Energy for Health [24], 2024










