





The figures leave no room for doubt: for ASA, the first quarter of 2022, compared to the same period of 2021, continues to show growth, both in terms of turnover and in terms of the devices produced.
This is the result of a well thought-out commercial strategy aimed at consolidating existing businesses while developing high-potential markets.
“We have been achieving these objectives thanks to a solid policy of working alongside our long-term partners, to whom we offer continuous support in planning activities and new functional products and technologies to gain a leading role in the market. Instead, in countries we are approaching for the first time, we have been focusing on supporting daily information and training activities to promote the dissemination of our technologies and therapies”.



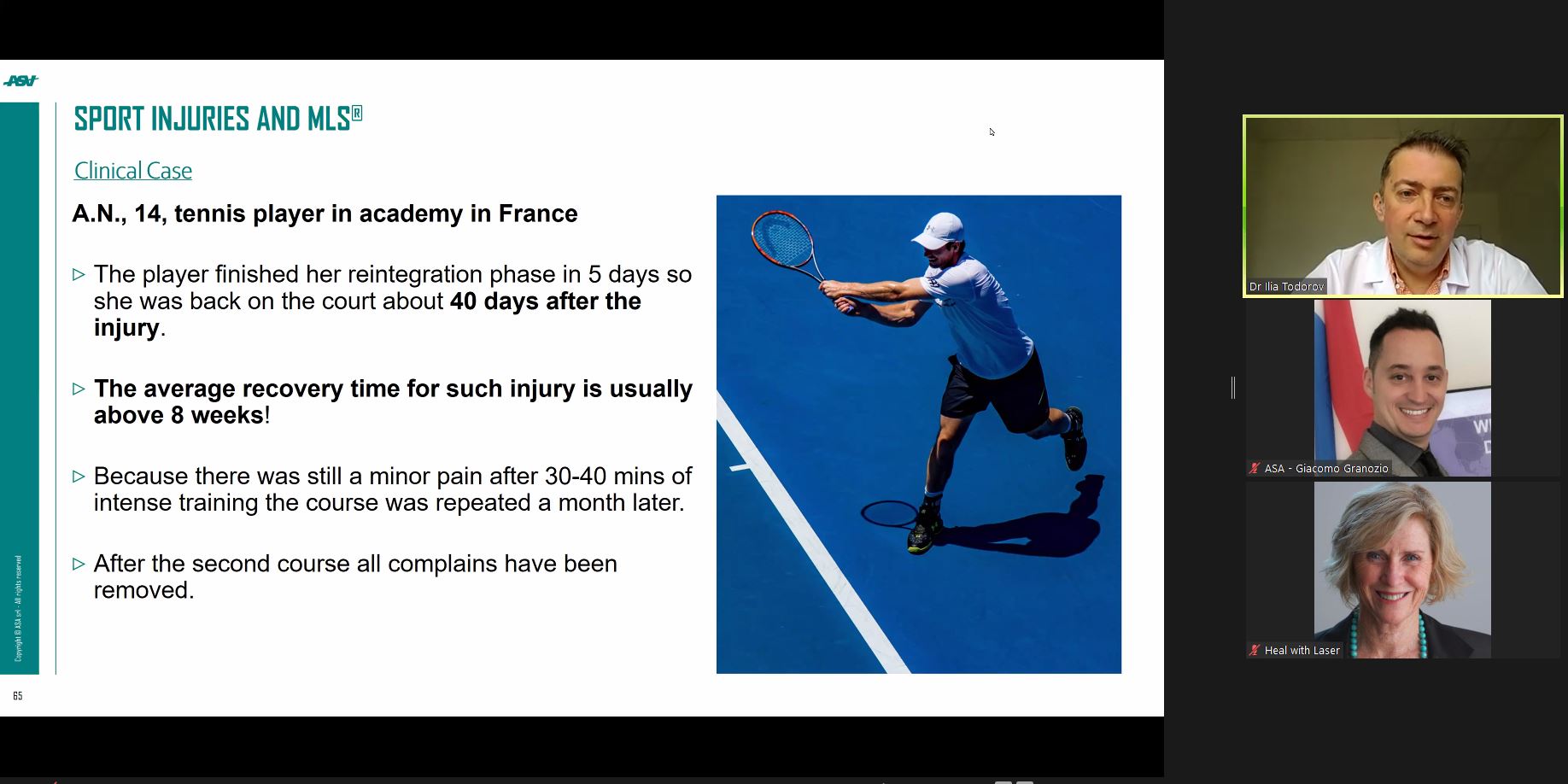


And during the first three months of the year, these therapies confirmed their growth and worldwide distribution.
The complex global socio-economic situation, together with the post-pandemic implications, has had a tangible impact on the company, but does not limit development projects.
“Pressure on prices, difficult-to-manage procurement schedules for components and packaging and significant increases in energy and transport costs are some of the critical issues we have to deal with on a daily basis. Unfortunately, we do not expect the situation to become stable any time soon, so we remain flexible and responsive to individual markets with very different commercial and training needs, but all equally relevant. Our distributors are aware of the situation and they are not quitting; they know that business is not only about the product: relationships and common visions are essential to win”,
concludes Federico Castellani - ASA Sales Manager.









L'accesso alla visualizzazione dei prodotti e al materiale informativo è riservato agli operatori del settore in ottemperanza alla legislazione vigente. ASA richiede di qualificarsi come operatore del settore per procedere con la navigazione.
Decreto Legislativo 24 febbraio 1997, n°46 Articolo 21
1. E' vietata la pubblicità verso il pubblico dei dispositivi che, secondo disposizioni adottate con decreto del Ministro della Sanità, possono essere venduti soltanto su prescrizione medica o essere impiegati eventualmente con l'assistenza di un medico o di altro professionista sanitario.
2. La pubblicità presso il pubblico dei dispositivi diversi da quelli di cui al comma 1 è soggetta ad autorizzazione del Ministero della Sanità. Sulle domande di autorizzazione esprime parere la Commissione di esperti prevista dall'articolo 6, comma 3, del decreto legislativo
30 dicembre 1992, n. 541, che a tal fine è integrata da un rappresentante del Dipartimento del Ministero della Sanità competente in materia di dispositivi medici e da uno del Ministero dell'Industria, del commercio e dell'artigianato.
Some of the contents of this website cannot be disclosed in the USA and its territories and possesions, for regulatory reasons. If you are a US resident, please click on the button here below and access ASA's distributor website for North America.