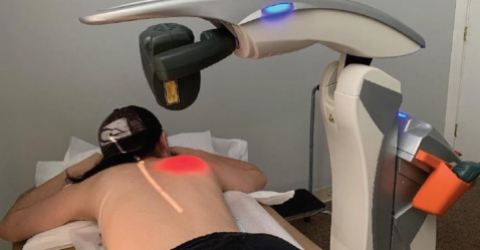
Temporomandibular pain: have you tried laser therapy?
Are you a woman between 20 and 40 years old? The possibility that you may suffer from temporomandibular disorders is a very real risk. Recent studies have confirmed that about 5-12% of the population has problems with their chewing muscles, temporomandibular joints (TMJ) and other anatomical structures of the face, neck and skull, with a significant majority in the female population. TMJ in particular is up to four times more common in women than in men. The symptoms, on the other hand, starting with the main one - pain - and those that accompany it: limitation of the movement of the jaw and noises during its use, do not differ by gender.
Where the causes of these disorders and their mechanism of action are still uncertain - they are however believed to be multifactorial (biomechanical, neuromuscular, biopsychosocial and biological), ed - there are no doubts about the difficulty in their management which, precisely because of its complexity, requires the synergistic intervention of various dental and neurological professionals and specific therapies. In order to find a solution that allows restoring full functionality and a good quality of life to sufferers, it is not uncommon for psychosocial and behavioural interventions to be combined with specific devices (occlusal bite splints), drugs (pain-killers, benzodiazepines, muscle relaxants, etc.), physical therapy (laser, ultrasound, transcutaneous electrical stimulation of the nerves - TENS - and microwaves), therapeutic exercise and manual therapy, occlusal adjustment and surgery.
In this multidisciplinary intervention plan, laser therapies have produced impressive results. This is confirmed by several scientific studies centred on the treatment of temporomandibular dysfunction and craniofacial pain that highlight how the application of MLS® Laser Therapy and of Hilterapia® have brought concrete and long-lasting benefits.
DTM & Laserterapia MLS®
Research by Manfredini et al (A comparison trial between three treatment modalities for the management of myofascial pain of jaw muscles: A preliminary study), for example, proved that the use of MLS® Laser Therapy significantly improved pain levels on the VAS scale and the muscle index of the Craniomandibular Index at 6 months’ follow-up in patients with myofascial pain of the maxillary muscles. The study conducted by Rosswag et al (Evaluation of the effect of MLS® Laser Therapy and Ora-GuardTM splint in the treatment of temporomandibular joint disease) verified how MLS® combined with the occlusal bite splint produces a greater improvement in the range of motion for patients and a significant reduction in pain compared to the use of the bite splint alone. The advantages of MLS®, Laser Therapy, according to the study by Ahmad et al (Effect of conventional therapy and low-level laser therapy on pain and limitations of daily functions in patients with temporomandibular joint dysfunction), are also enjoyed by sufferers of myofascial pain syndrome from temporomandibular joint dysfunction. Treated in parallel with conventional and laser therapy, these patients reported a significant enhancement of their pain threshold to pressure and a decrease in pain-related limitations in daily functions.
DTM & Hilterapia®
Where the positive effects of the application of LLLT and high-power lasers in the treatment of these disorders are therefore known, scientific evidence confirming the efficacy of high-intensity laser therapy (Hilterapia®) in this field has not been available until recently. Recently, however, three different studies have been carried out by Ekici et al which centred on the use of the HILT® pulse on patients with temporomandibular disorders with promising results. The studies were carried out by randomly assigning patients to a HILT® group or to one or more control groups such as Placebo-HILT® with exercises, occlusal bite splints or other physical therapies (TENS and ultrasound). The results obtained showed that following the application of the ASA therapy, at 4 weeks from the follow-up all the parameters assessed (maximum autonomous and assisted opening of the mouth, pain, function, quality of life and disability) recorded a marked improvement, even after 12 weeks from the start of the treatment. All without contraindications or adverse events.
Finally, it was found that, compared to the control group, the monitored parameters were extremely positive for the HILT® patients when compared with those of the patients subjected to Placebo-HILT®, TENS and exercise. An even greater improvement was found when comparing the data obtained from treatment with ultrasound or occlusal bite splint.
Therefore, whether it is MLS® or Hilterapia®, the use of lasers for those who have to deal with temporomandibular disorders has valuable repercussions both in restoring impaired functionality and in reducing pain.

“[...] HILT is a highly effective, non-invasive therapeutic method for patients with myogenic TMD.”
Effectiveness of high-intensity laser therapy in patients with myogenic temporomandibular joint disorder: A double-blind, placebo-controlled study
Ö. Ekici, Ü. Dündar, M. Büyükbosna
Journal of Stomatology, Oral and Maxillofacial Surgery, 2022

“[...] The healing effect of pulsed Nd: YAG laser therapy was significantly higher than TENS in patients with DDWR. Therefore, HILT should be a priority option over TENS therapy in patients with disc displacement.”
Comparison of the Efficiency of High-Intensity Laser Therapy and Transcutaneous Electrical Nerve Stimulation Therapy in Patients With Symptomatic Temporomandibular Joint Disc Displacement With Reduction
Ö. Ekici, Ü. Dündar, M. Büyükbosna
Journal of Oral and Maxillofacial Surgery, 2022

“[...] OS, US, and HILT are effective treatment methods for pain and functional jaw movements in patients with DDWR. HILT, a new method, can be an alternative treatment in cases of TMD.”
Evaluation of the efficiency of different treatment modalities in individuals with painful temporomandibular joint disc displacement with reduction: a randomised controlled clinical trial
Ö. Ekici, Ü. Dündar, G. Deste Gökay, M. Büyükbosna
British Journal of Oral and Maxillofacial Surgery, 2022