Clinical research: MLS® laser treatment to alleviate diabetic neuropathy pain
The clinical research* conducted by Dr. Yosifova, as published in the Journal of IMAB, highlights the promising role of ASA's M6 robotic laser in mitigating neuropathic pain associated with diabetic sensorimotor neuropathy.
Understanding Diabetic Neuropathy
Neuropathic pain affects a significant proportion of patients with diabetes, with a prevalence ranging from 20-50%, escalating to 60% in those with diabetic neuropathy. The severity of neuropathic pain is often dissociated from nerve damage, manifesting as tingling, burning sensations, sharp stabbing pain, and electric shock-like sensations.
Study Population and Methodology
Sixty-nine patients with type 2 diabetes and painful diabetic neuropathy were randomly assigned:
- 41 to the treatment group (MLS® Laser Therapy)
- 28 to the control group (Sham-Laser).
Patients continued to take diabetes drugs as previously prescribed.
MLS® was applied as monotherapy and comprised 9 sessions over 3 weeks, administered every other day, targeting specific points on the feet using the M6 robotic laser.
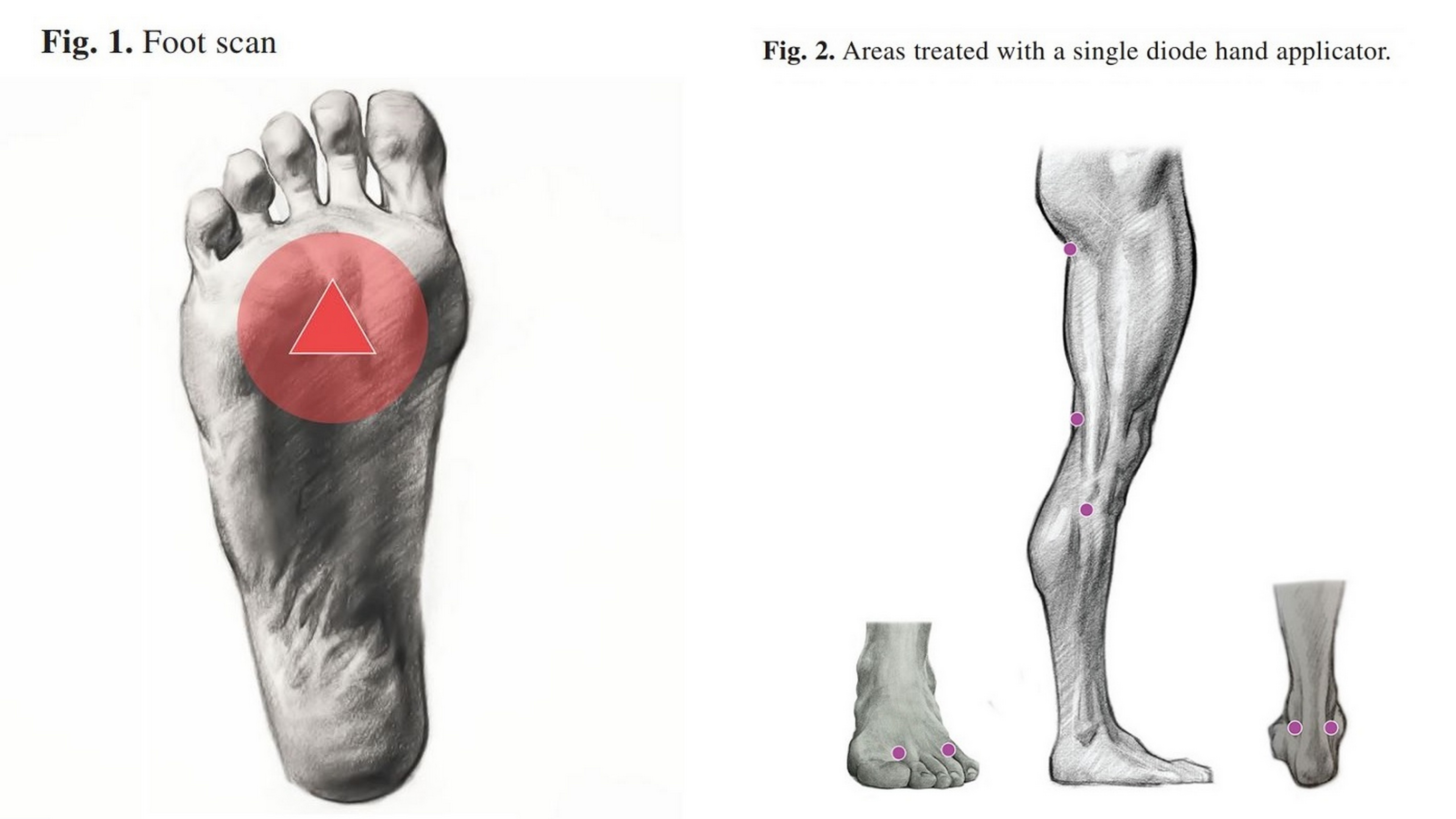
Results that Speak Volumes
The evaluation took place during the baseline visit (T0), after the conclusion of the treatment cycle (T1 - 21 days later), and during the follow-up visit, 90 days after the initial treatment (T2).
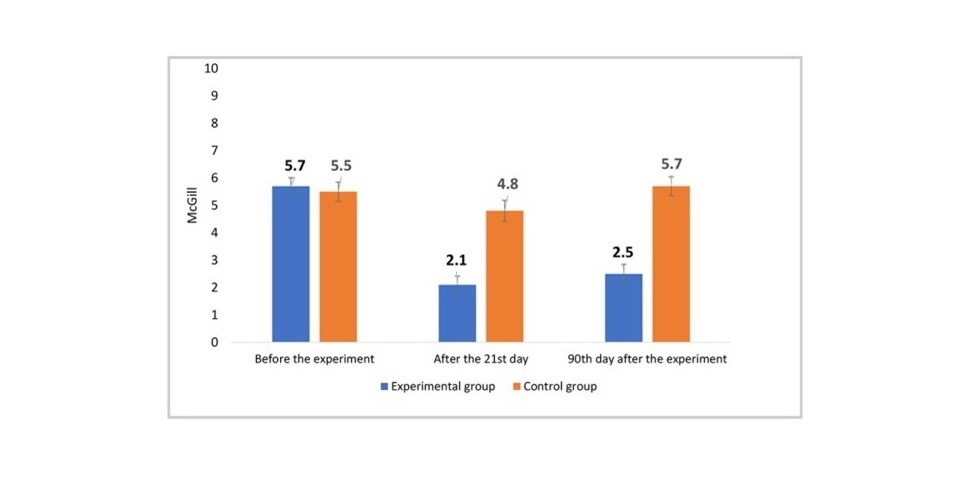
- The treatment group exhibited a substantial reduction in pain, as measured by the visual analogue scale (VAS), from 5.7 to 2.1 at the end of the treatment cycle.
- Conversely, the control group demonstrated modest changes in pain levels, reinforcing the unique efficacy of ASA's MLS® Laser Therapy.
Conclusion: A Ray of Hope for Neuropathic Pain Sufferers
The two groups demonstrated homogeneity as concerns the duration of the diabetic neuropathy, the demographic and anthropometric indicators, the therapeutic interventions previously administered to treat diabetes, and the glycated hemoglobin levels.
- MLS® Laser Therapy significantly reduced neuropathic pain by -63.2%, in stark contrast to the -12.7% observed in the placebo-control group.
- The positive effects persisted in the medium to long term, with a -56.1% reduction at the follow-up visit (T2), compared to a +3.7% increase in the placebo-control group.
MLS® Laser Therapy could be a valid non-pharmacological therapy complementary to standard therapy in patients with painful diabetic peripheral neuropathy.

Effects of MLS-Laser on neuropathic pain in diabetic sensomotor neuropathy
L. Yosifova, E. Vladeva, M. Siderova
Journal of IMAB, Jul-Sep;29(3):5079-5084, 2023
Beyond pain reduction
The previous article was extracted from Dr. Yosifova's doctoral thesis** but the PhD thesis presents additional clinical outcomes beyond the pain reduction assessment, including the restoration of sensitivity to stimuli such as vibration, touch, and temperature, the alterations in glycated hemoglobin (HbA1c), and the improvements in peripheral nerve function.
Sensitivity restoration
- Vibrational Stimulus: using a Rydel-Seiffer tuning fork, the treatment group demonstrated a significant and long-lasting increase in vibration sensitivity across all measurement sites, outperforming the control group.
- Tactile Stimulation: employing the Semmes-Weinstein monofilament test, the treatment group showcased notable bilateral improvement in touch sensitivity, a trend maintained through to the 90-day follow-up, unlike the control group.
- Thermal Stimulus: using a thermal discriminator, the treatment group exhibited considerable enhancement in responsiveness to thermal stimuli, indicating a lasting improvement compared to the control group.
Glycated hemoglobin
Despite the lack of significant change in glycated hemoglobin levels, the study suggests that the observed improvements are not solely attributable to changes in diabetes control but rather affirm the efficacy of MLS® Laser Therapy.
Neurophysiological examination
- Sensory Fibers (Sural Nerve): significant increases in SNAP amplitude (sensory nerve action potential), conduction velocity (CV), and decreased distal latency time (DL) were observed in the treatment group, showcasing positive impacts on the sensory fibers compared to the control group.
- Motor Fibers (Tibial and Peroneal Nerve): although no significant differences were found in the total motor action potential (SMAP), the treatment group displayed increased CV and decreased DL, emphasizing positive effects on the motor fibers compared to the control group.
What are the results achieved?
- MLS® Laser Therapy improved superficial and deep sensation of the lower limbs, pain threshold, and nerve function in patients with diabetic sensorimotor neuropathy.
- Electroneurographic parameters of the peripheral sensory and motor nerves of the lower limbs showed statistically significant improvement.
- The therapy resulted in a reduction of neuropathic pain and an improvement in both superficial and deep sensitivity of the lower limbs.
The results indicate that MLS® Laser Therapy has immediate, cumulative, and long-lasting effects on sensory evaluation, with a better effect on the sensory nerves. Furthermore, MLS®was well-tolerated by the patients and did not cause any discomfort or adverse reaction.
** STUDY OF THE EFFECT OF HIGH ENERGY LASER IN DIABETIC SENSORIMOTOR NEUROPATHY
https://eprints.mu-varna.bg/handle/nls/2736?locale-attribute=bg









