World PT Day 2023: arthritis backed into a corner with laser therapy
How to treat arthritis by acting on inflammation and managing the patient's pain with effective results: this is the theme of the 2023 edition of World PT Day (8 September) which focuses not only on the disease of which there are over 100 types, but on the physiotherapist’s role as well. He or she, in fact, has the task of supporting and assisting those who suffer from this through a series of treatments aimed at preventing damage to the joints and at alleviating suffering.
Managing Arthritis: targeted treatments to relieve pain and aid recovery
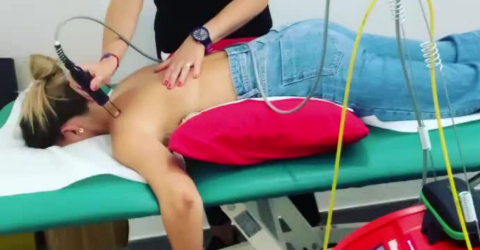
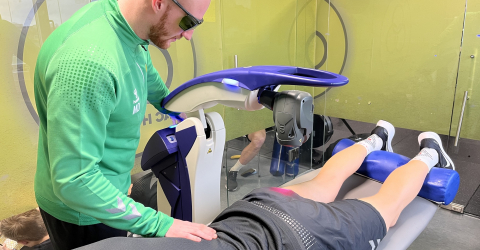
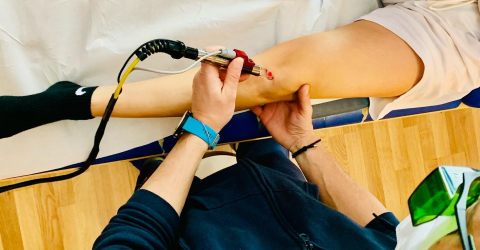
Room therefore not only for medications, but also for manipulations and therapeutic solutions, such as for example MLS® Laser Therapy and Hilterapia® which, thanks to their analgesic, anti-inflammatory, and anti-oedema effects, are useful for promoting tissue repair and regeneration processes and for overcoming the painful symptom. But that's not all: the two therapies are confirmed to be valuable in limiting and recovering impaired mobility and in supporting the skeleton, effectively promoting the recovery of functionality.
Arthritis: a progressive disease with implications for daily life
If not diagnosed in time and properly treated, arthritis progressively worsens and can cause significant difficulties even in carrying out common activities at work and during free time. The latter is a problem that the disease, characterised by pain, stiffness, heat and redness of the affected joint, weakness, and loss of muscle tone, shares with osteoarthritis.

MLS® Laser Therapy and Hilterapia®: an innovative approach to managing arthritis
There are several scientific research studies that underline how laser therapy can help put a stop to pain, stiffness and inflammation. An example is the study “Short-Term Efficacy of High-Intensity Laser Therapy in alleviating pain in patients with knee osteoarthritis: a single-blind randomised controlled trial” which, aimed at assessing the efficacy of Hilterapia® on pain reduction in patients with knee arthritis, has recognised its value as a conservative treatment, particularly advantageous for elderly patients and for those at high risk of surgery due to comorbidities. A further certificate of merit to the HILT® pulse also comes from the research “Effectiveness of high-intensity laser therapy in the management of patients with knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials”. Based on a systematic review and a meta-analysis of 6 randomised controlled trials to assess the efficacy of therapy in patients with knee osteoarthritis, the analysis concluded that the contribution of “HILT® on pain, stiffness, and function in patients with knee osteoarthritis is promising”. MLS® Laser Therapy also offers a significant contribution to putting a stop to the disease. In fact, according to the study “Efficacy of class IV diode laser on pain and dysfunction in patients with knee osteoarthritis: a randomized placebo-control trial”, its application combined with physical exercises reduces pain and the WOMAC (Western Ontario and McMaster Universities Index of Osteoarthritis) much more than the use of a placebo laser associated with physical activity.
Beyond laser therapy: nutrition and movement as key components
Where laser therapy therefore plays a primary role in treating arthritis and osteoarthritis, in terms of prevention but also of cure, two other factors are not secondary: nutrition and movement. A healthy and balanced diet is in fact essential to supply the body with all the necessary nutrients and to counteract overweight, the primary cause of excess pressure on the joints of the hips, knees, ankles, and feet. Regular physical exercise, on the other hand, counteracts the progress of arthritis since, in addition to reducing joint pain and stiffness, it improves range of motion and increases muscle strength and energy.