Efficacy of Hilterapia® and physical exercise in the treatment of Cervical Radiculopathy: Results of a clinical study
Cervical Radiculopathy is a condition characterised by the compression of the cervical spinal nerve roots, which manifests with painful symptoms and functional limitations.
A recent research, “Clinical Efficiency Of High-Intensity Laser Therapy In Patients With Cervical Radiculopathy: A 12-Week Follow-Up, Randomised, Placebo-Controlled Trial.” investigated the combined application of Hilterapia® (with the Hiro 3.0 device) and physical exercise in the treatment of Cervical Radiculopathy (CR).
The study: method and evaluation
The study involved a sample of 90 patients diagnosed with cervical disc herniation. Participants were randomly assigned to one of three treatment groups: Hilterapia® combined with exercise (HILT + EX), placebo combined with exercise (Placebo + EX), and exercise alone (EX only).
Several parameters were assessed to measure the effectiveness of the treatment, including:
- Assessment of pain intensity using the Visual Analogue Scale (VAS)
- Assessment of neuropathic pain using the PainDETECT Questionnaire (PD-Q)
- Assessment of radicular pain using the Cervical Radiculopathy Impact Scale (CRIS)
- Assessment of the cervical range of motion, including flexion, extension, right and left lateral tilt, right and left rotation
- Assessment of functional activity using the Neck Disability Index (NDI) Questionnaire
- Assessment of health-related quality of life using the 36-item Short Form Health Survey (SF-36)
The treatment protocol with Hilterapia® involved a total of 20 sessions, with a frequency of 5 sessions a week for 4 weeks.
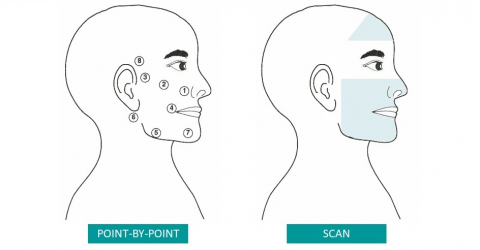
Each session included three phases (scan, sensitive trigger points, slow scan) and had a total duration of 20 minutes.
During each session, a total energy of 2632 J was applied.
The results: the role of Hilterapia®
Individuals were evaluated by an independent investigator, who was unaware of the treatment group, at the start of the study (T0), after 4 weeks (T1 - end of treatment) and after 12 weeks (T2) from the start of the treatment cycle.
The results highlighted that, although the placebo effects influenced the results, the group that underwent the combination of Hilterapia® and physical exercise (Hilterapia® + EX) showed significant improvement in pain reduction (VAS), in the control of neuropathic pain (PD-Q), in the impact of radicular pain (CRIS) and in neck disability (NDI) in both the short and medium term.
Furthermore, the Hilterapia® + EX group demonstrated a clear superiority over the other groups in the medium term, as concerns the increase of the cervical range of motion in the directions of extension (p=0.005), right rotation (p=0.013) and right lateral tilt (p<0.001).
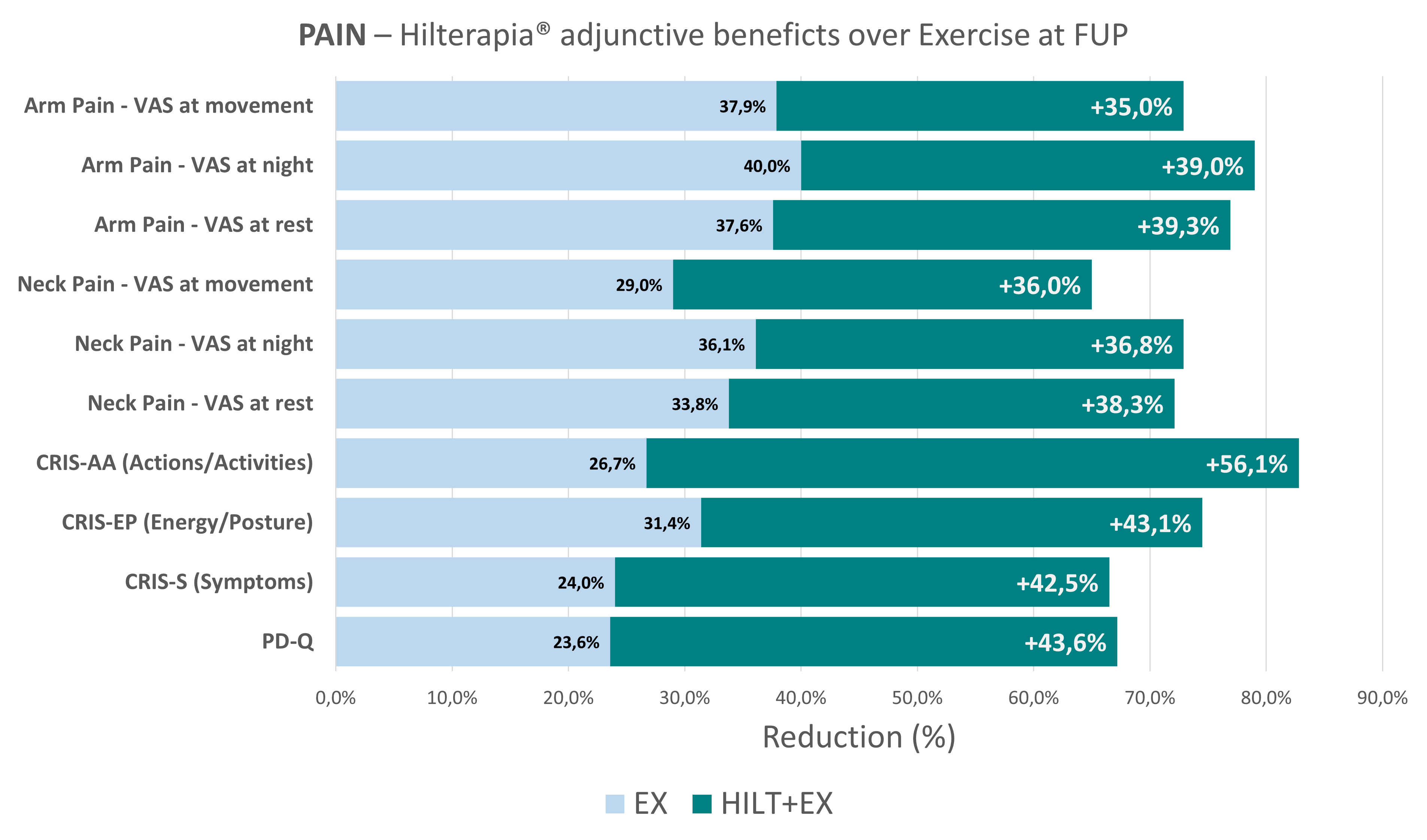
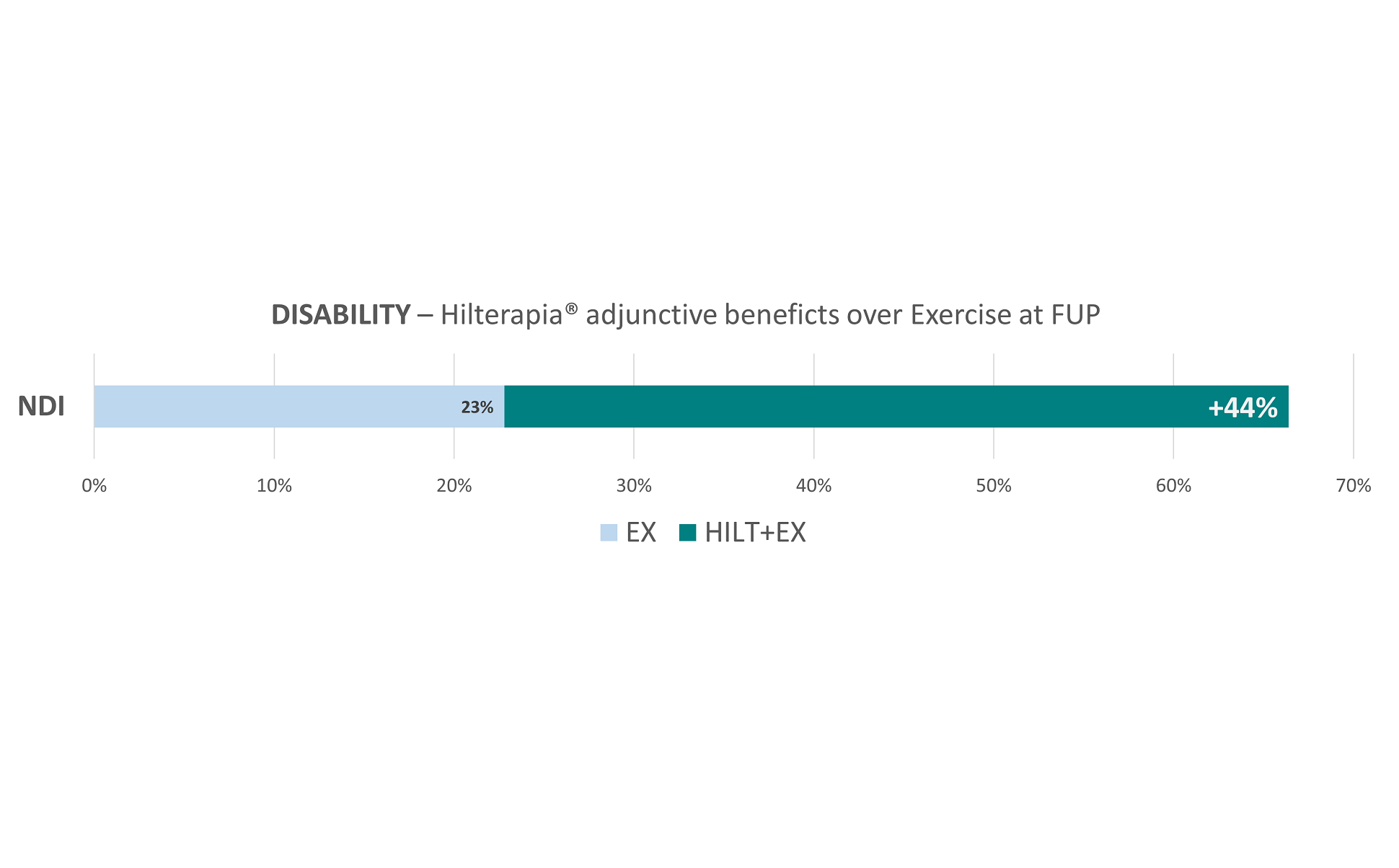
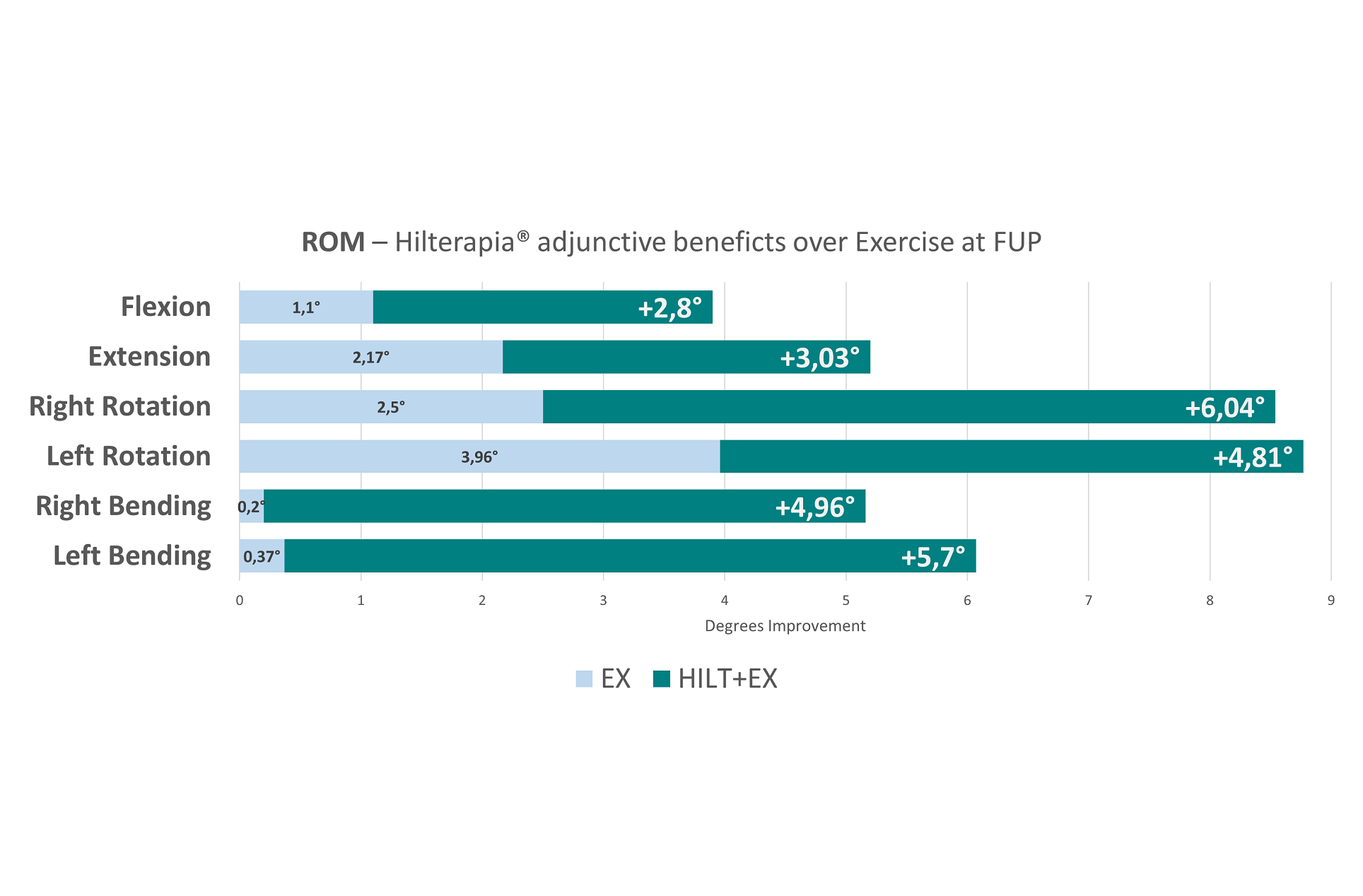
Interestingly, the Hilterapia® + EX group also reported significant improvement in all SF-36 sub-parameters compared to the other two groups. It should be emphasised that no adverse effects were found during treatment and follow-up, neither with Hilterapia®, nor with placebo, nor with physical exercise.
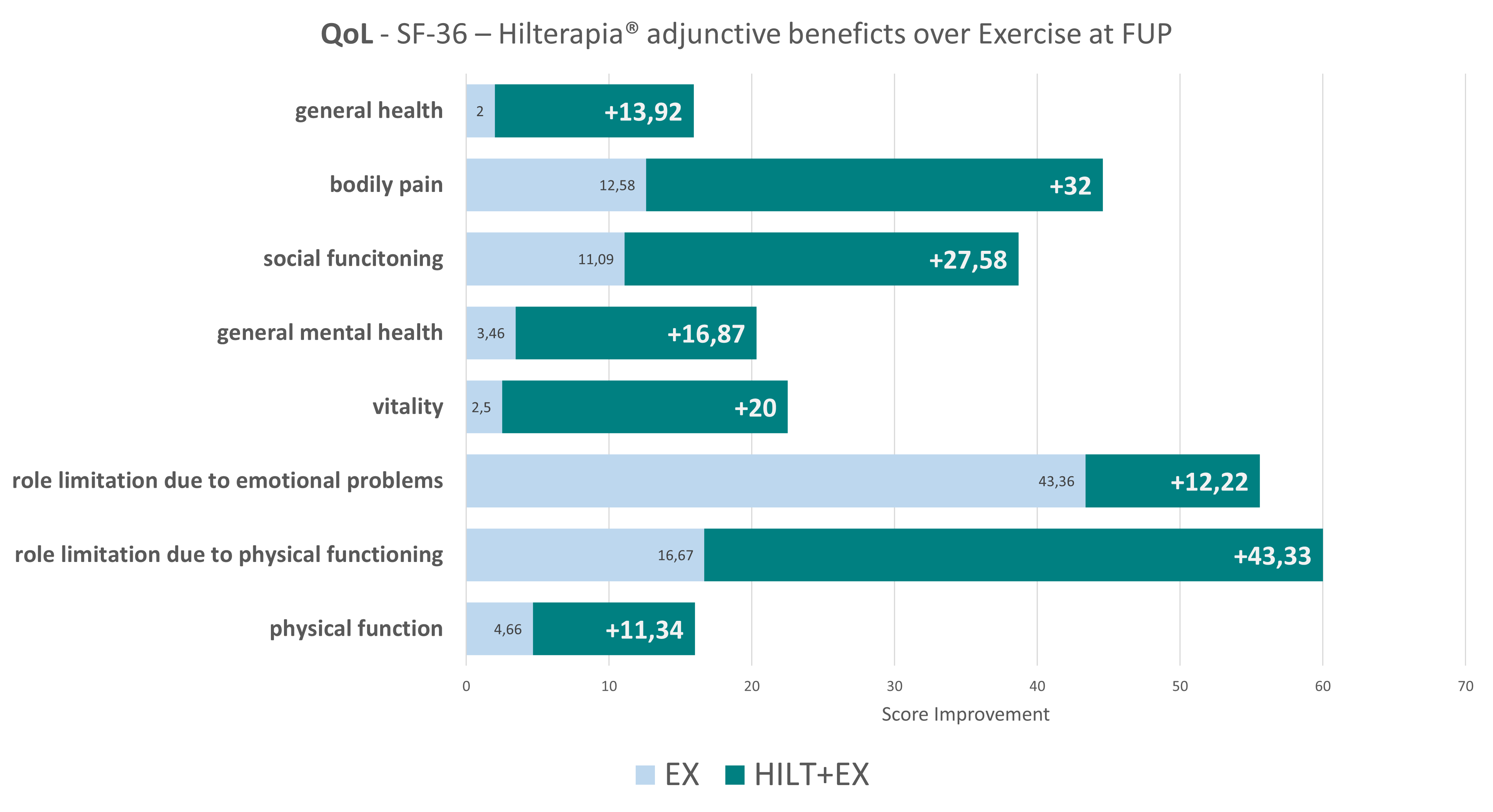
In conclusion, the association between Hilterapia® and physical exercise proved to be superior to placebo or physical exercise alone in reducing radicular pain and improving the functionality and quality of life of patients suffering from Cervical Radiculopathy. It is important to underline that these benefits have been maintained in the medium term.
The key message emerging from this study is that Hilterapia®, in combination with physical exercise, can be considered an effective treatment for patients with chronic symptoms of Cervical Radiculopathy.
The results obtained provide solid evidence of the efficacy of this non-invasive therapy in improving the well-being of patients suffering from this painful condition.