How to put a stop to tendinopathies and tendinitis? No limits motorcyclists
Getting back on the road when the muscles throw a fit and the pain accelerates? Thanks to laser therapy you can! Easy(er) life for motorcyclists who deal with tendinopathies, tendinitis, and back pain from insufficient training.
Motorcyclists are well aware of this: getting on the bike requires a basic training to avoid muscular repercussions on the upper and lower limbs and back with painful consequences that then take a long time to be solved and as much time to recover. If you belong to the category of weekend riders but also of athletes in the super-training phase and you find yourselves facing too much pain due to excessive use of muscles and joints, to get back on track quickly you need to find the right solution.
The laser therapy
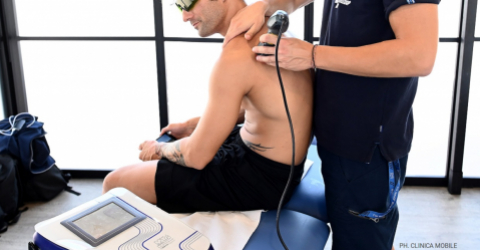
Professional motorcyclists set the example and, in addition to physiotherapists, they rely on Laser therapy because, by stimulating biochemical reactions and vasodilation, increasing the supply of oxygen and nutrients, it facilitates the resolution of the inflammatory process and the reabsorption of oedema. But there’s more! Thanks to its analgesic action, pain too is alleviated quickly, and getting back on the road is child’s play.
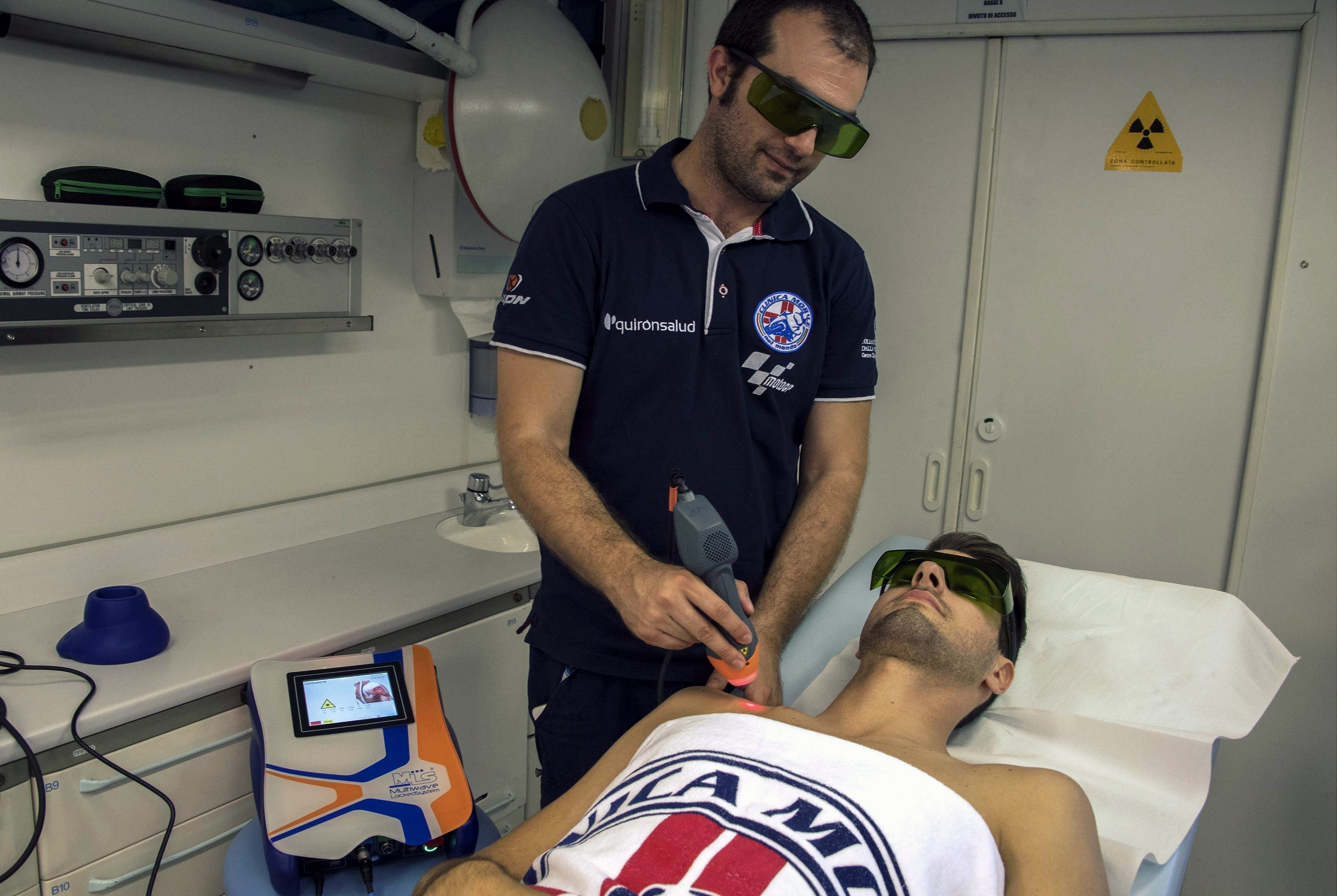
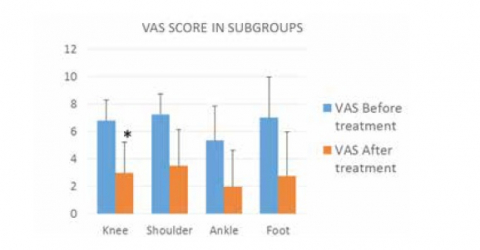
The most recurrent problems in the motorcycle field, except for traumatic pathologies, are those from overuse, first among them insertional tendinopathies. To be able to better manage the factors that cause them, such as neovascularization, through anti-oedema and anti-inflammatory stimulations, Laser Therapy has proven to be a particularly effective support: effective, safe, and quick in application times as well as in obtaining results.
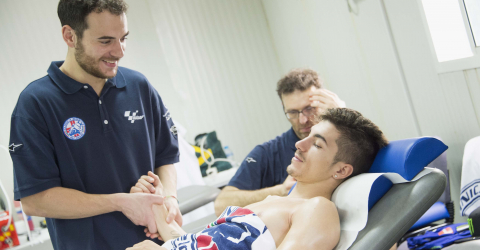
Morgan Bignami, physiotherapist at the Clinica Mobile, a point of reference for MotoGP riders.
If Laser therapy is used to solve the damage once it is done, prevention is also essential. Training is the rule: make room for swimming, running, and lifting weights to strengthen the trunk, arms, and legs, and for targeted stretching sessions for the forearm and wrist to keep the onset of risks related to repeated excessive stresses under control.