Thailand: 4 Hilterapia® Seminars at reference hospitals
Spotlighting the excellence of Hilterapia® by underlining its uniqueness through a targeted focus on its technical features, scientific evidence and clinical outcomes. This was the objective of the 4 seminars themed “Effect of High Intensity Laser Therapy on joint diseases in acute and chronic conditions” organised by ASA in collaboration with its Thai distributor IMS in 3 hospitals between Bangkok and Chiang Mai from 28th February to 3rd March: Bangkok Hospital, Maharaj Nakorn Chiang Mai Hospital, where the Faculty of Medicine of the Chiang Mai University is located, and Siriraj University Hospital, which includes the Faculty of Medicine of Mahidol University.



“To emphasise the value of Hilterapia® - Giacomo Granozio, ASA Area Manager, explains – we led off from a solid theoretical base centred on its biological effects, detailing the results of all the scientific research carried out by ASAcampus and focused on the repair and regeneration effect of the therapy. This premise, able to define and diversify our technology, was the impetus for the speech by Doctor Wolfang Gruther - MSc, Univ.- Lektor Medical University of Vienna – a long-time user of HIRO TT”.
The German authority shared his experience, illustrating advanced methods of use of the therapy.
A structured path that was able to lean on the direct involvement of the participants, who proactively asked questions and requested specific indications, particularly during the hands-on session.
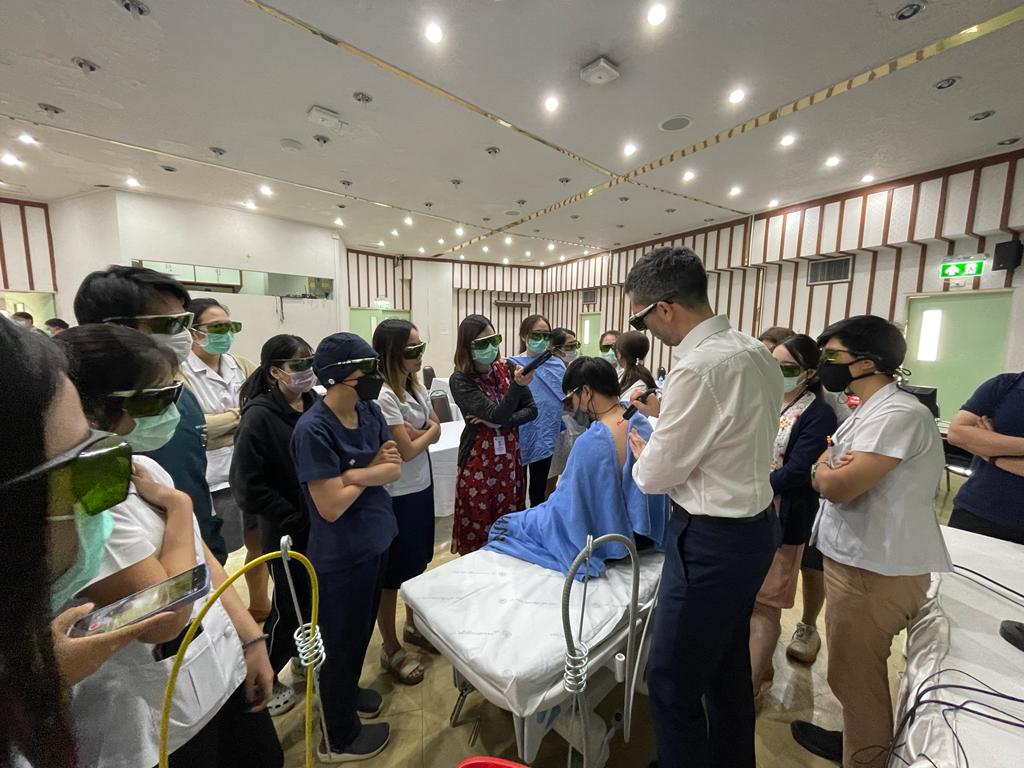



“Two sessions on different days were organised at the Bangkok Hospital: the first one concentrated on the theoretical aspect whilst the second, a few days later, on the practical one, attended by 70 people online and by 50 in person, who were able to rotate in the 5 stations equipped with HIRO TT, HIRO 3.0 and SH1. An organisational complexity that was managed extremely efficiently by our local partner”.
The success of the training initiatives was confirmed not only by the considerable number of attendees, but also by the feedback received at the end of the meetings.
“We are very satisfied with the results achieved and with having been able to organise a format that has all the potential for becoming a valid tool to be reproposed to other markets”, Granozio concludes.














