Research detects the anti-inflammatory effect of MiS on cells and molecules
ASAcampus is constantly at the forefront in developing new research. The latest is “Effect of NIR Laser Therapy by MLS‐MiS Source on Fibroblast Activation by Inflammatory Cytokines in Relation to Wound Healing” che si which aimed to study the anti-inflammatory activity of MiS, the ASA device capable of combining the synchronised action of MLS® Laser Therapy with the pulse power of Hilterapia®, from the cellular and molecular point of view.
The in-vitro study was carried out on human dermal fibroblasts exposed firstly to a mix of inflammatory cytokines (IL‐1β and TNF‐α) which subsequently underwent treatment with MiS.
Three types of samples were analysed:
- the first concerned human fibroblasts stimulated with IL‐1β and TNF‐α for 48 hours which subsequently underwent 3 laser treatments (once a day for 4 consecutive days);
- the second envisaged a similar stimulation mix, but avoided exposure to the laser;
- the third neither underwent stimulation, nor laser.
After analysing the results, the researchers noted that the main pathways signalling inflammation were increased (or activated) by the mix of cytokines, whilst treatment with MiS lowered the levels, restoring basal levels, in a statistically significant manner. The same was noted when assessing the VEGF levels (the factor involved in neovascularisation of granular tissue).
The research also allowed analysing some of the most important cytoskeletal proteins which had modified their organisation after stimulation with cytokines, and returned to the basal distribution after treatment with MiS.
It can therefore be concluded that not only does the ASA device have an important anti-inflammatory effect, but it is also effective in controlling fibroblast activation induced by IL‐1β and TNF‐α, probably responsible for a detrimental effect on the inflammation’s evolution, which favours its persistence.
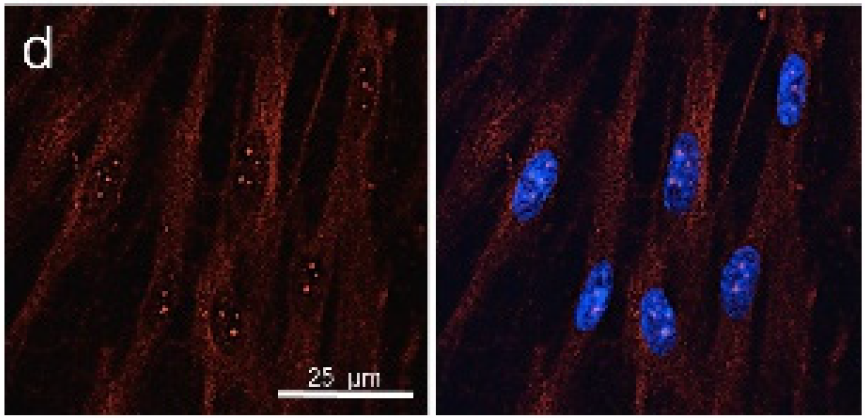
“Effect of NIR Laser Therapy by MLS‐MiS Source on Fibroblast Activation by Inflammatory Cytokines in Relation to Wound Healing”
S. Genah, F. Cialdai, V. Ciccone, E. Sereni, L. Morbidelli, M. Monici
Biomedicines 9, 307, 2021