Journal Of Inflammation Research: MLS® Laser Therapy and the consequences of interstitial pneumonia caused by COVID-19
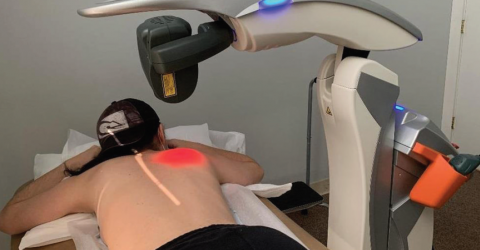
“When the covid pandemic hit us, I thought about the idea of using MLS® on patients affected by the virus. We see great results in acute inflammation resulting from joint damage and this was the natural connection.”
This was how the experience of Dr Scott Sigman began, orthopaedic surgeon in Boston and Chief Medical Officer at OrthoLazer Orthopedic Centers.
The publication in the Journal of Inflammation Research entitled “Evaluation of Adjunctive Photobiomodulation (PBMT) for COVID-19 Pneumonia via Clinical Status and Pulmonary Severity Indices in a Preliminary Trial” highlights – within a clinical study – the legitimacy of Dr Sigman’s intuition and the validity of MLS® Laser Therapy on patients suffering from interstitial pneumonia caused by COVID-19.
Among the researchers at the forefront are Dr Scott Sigman (Team Physician UMASS Lowell, member of the World Society of Sports and Exercise Medicine and of the Royal College of Surgeons in Ireland, founder and director of OrthoLazer Orthopedic Laser Centers) who was the first to use MLS® Laser Therapy in this field, and Dr Monica Monici, head of ASAcampus – Joint Laboratory with the Department of Experimental and Clinical Biomedical Sciences of the University of Florence.
The clinical study involved 10 patients, randomized into two groups of 5 people each: the first group (control group) was subjected to the standard protocol (oxygen supplementation, fluid and electrolyte balance, standard nursing care), while the second to the standard treatment and 1 MLS® Laser Therapy session per day for 4 consecutive days.
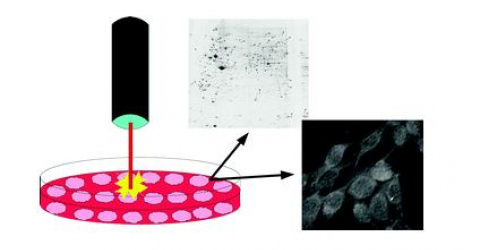
“Treatment with MLS® induces an increase in the anti-inflammatory protein NLRP10. It is a protein capable of inhibiting the production and release of inflammatory cytokines such as interleukin 1-β (IL-1β) and interleukin 18 (IL-18)’, explained Dr Monica Monici. ‘When they enter the circulation, IL-1β and IL-18 stimulate the production of other pro-inflammatory cytokines such as interferon-γ (INF γ), TNFα, IL-6, etc., resulting in a chain reaction that amplifies and extends the existing inflammation. Basically, by dampening the release of IL-1β and IL-18, the anti-inflammatory protein NLRP10, induced thanks to MLS®, is able to control and reduce the inflammatory reaction”. ("Effect of IR laser on myoblasts: a proteomic study")
Results
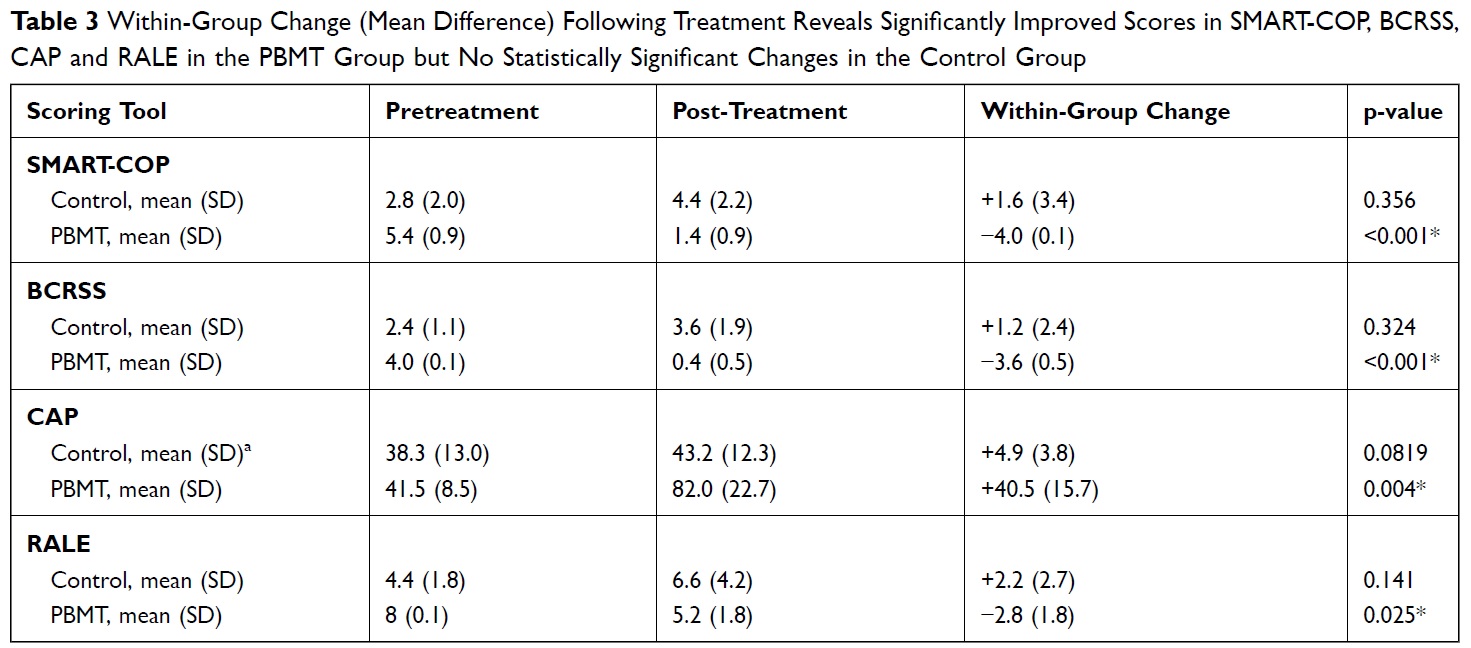
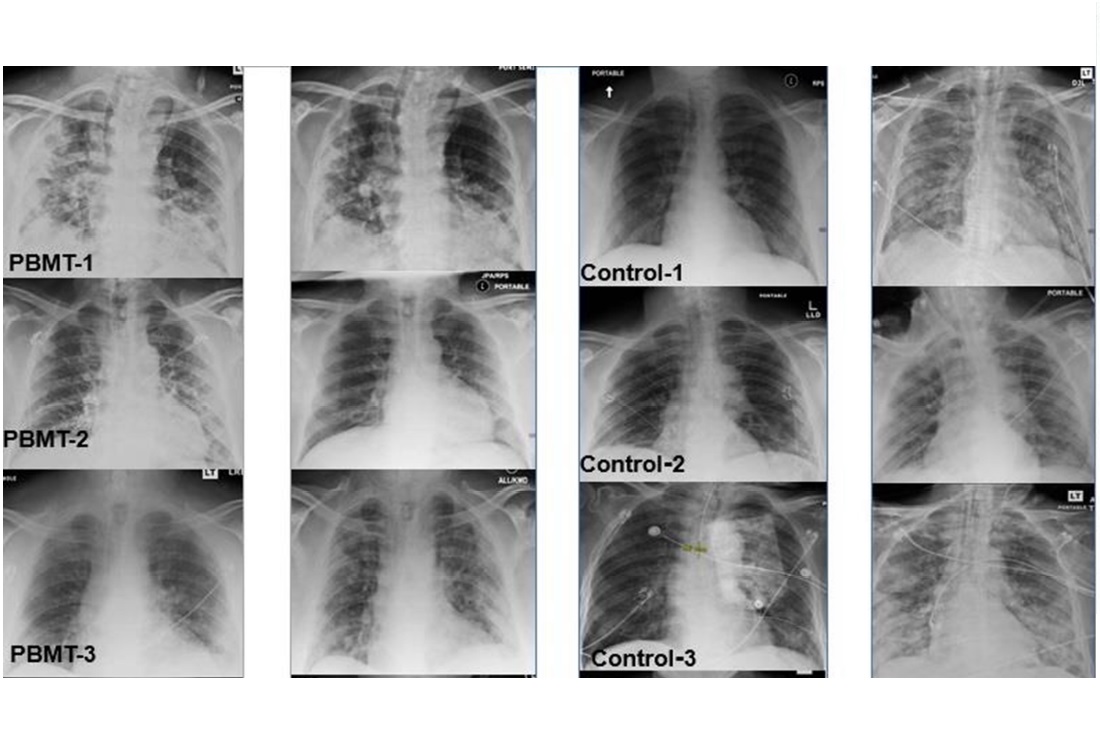
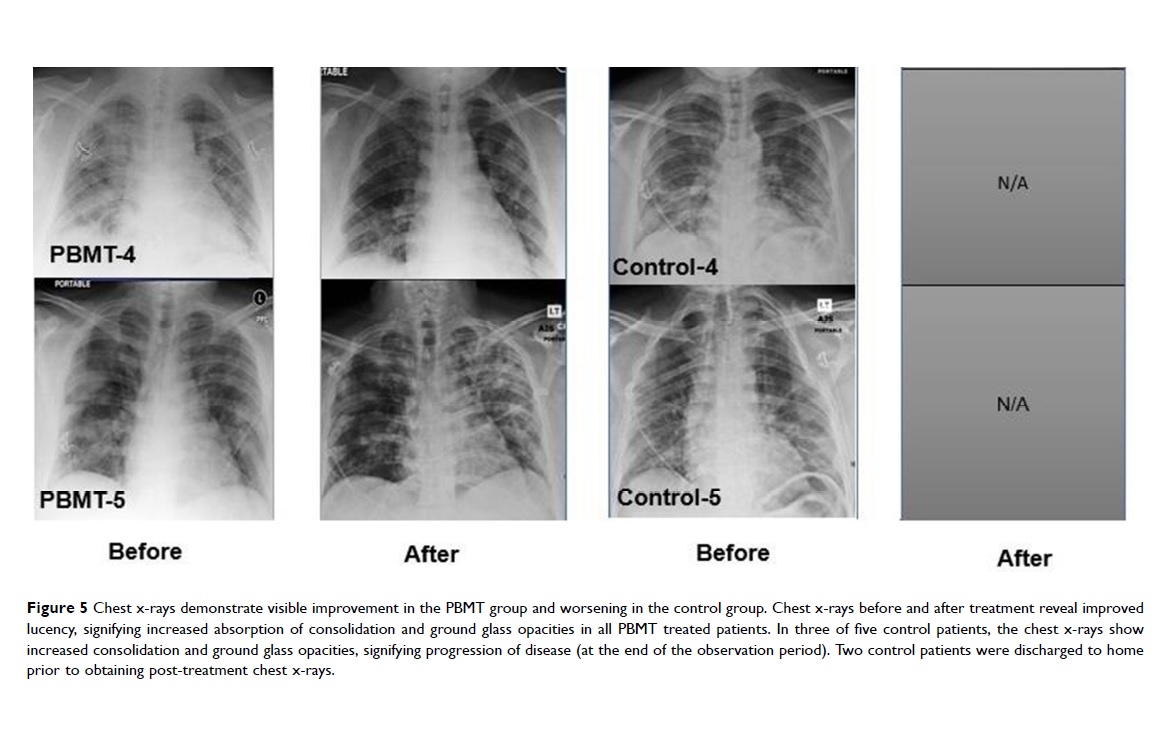
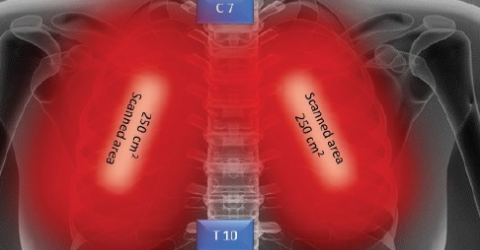
The recent publication in the Journal of Inflammation Research reports that the effects on the patients were measured through blood tests, chest X-rays, oxygenation monitoring, and through validated scores – subjective and objective – for pneumonia. The results actually showed that patients undergoing MLS® Laser Therapy:
- had a rapid recovery
- showed an improvement in pulmonary indices (score: SMART-COP, BCRSS, RALE and CAP)
- did not need intensive care or mechanical ventilation support
- did not report any posthumous consequences 5 months after treatment.
60% of the patients belonging to the control group, on the other hand, underwent intensive care with mechanical ventilation, recorded an overall mortality of 40% and, in the 5-month follow-up, 40% of cases had long-term sequelae.