Taipei: web & live congress for MLS®
The COVID-19 emergency and the mandatory social distancing have not stopped the initiatives of ASA and of its distributors who, thanks to the mixed “on site” and ‘on line” formula, continue to carry out meetings to analyse the company’s therapeutic solutions.
The most recent one was the “Taipei MLS® User Meeting” congress which took place at the Caesar Park in Taipei thanks to the cooperation of Gaia Genomics, our local partner.
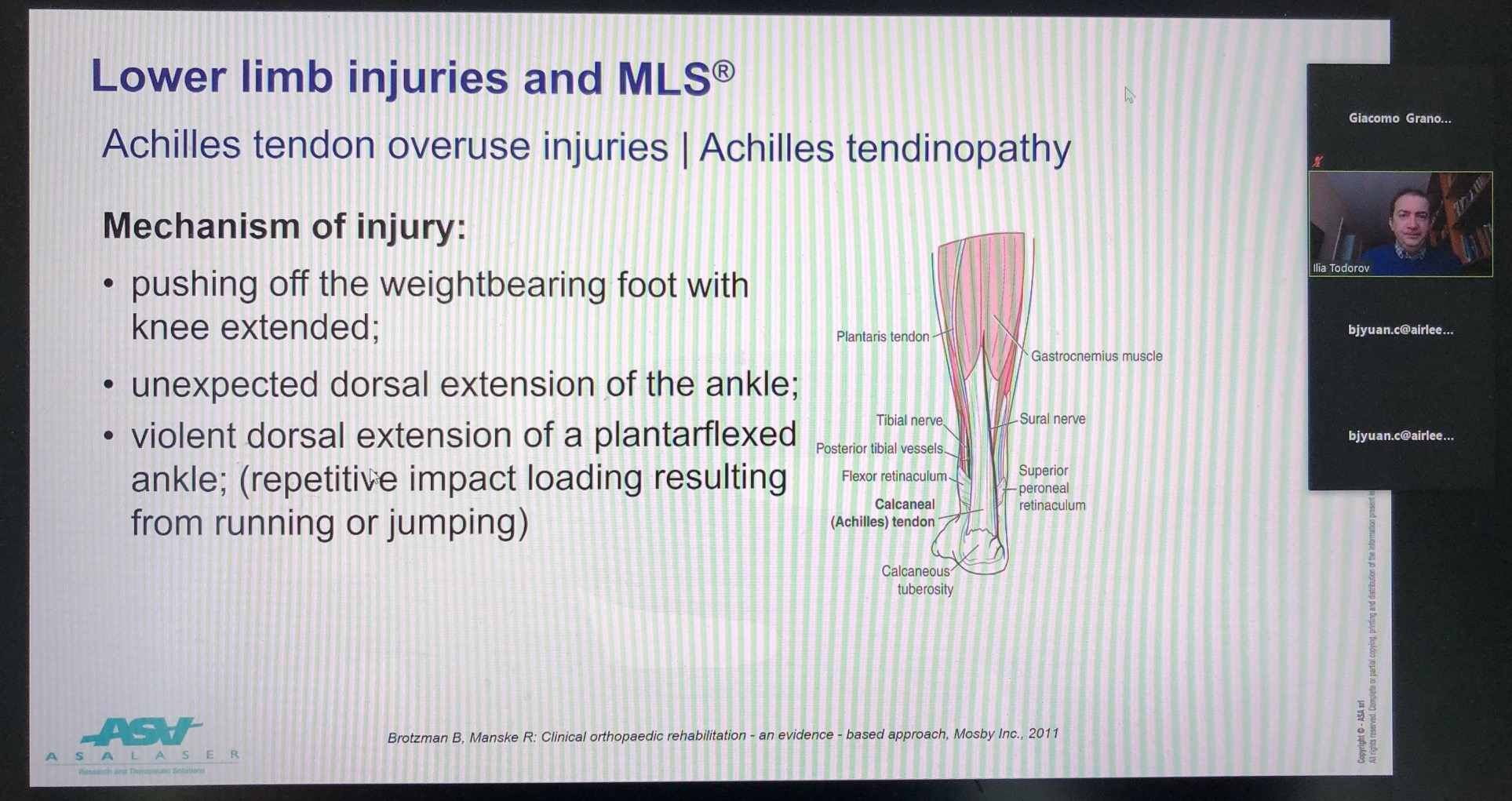
The meeting was physically attended by 4 specialists in the field of physiotherapy and rehabilitation who shared their clinical experience in using the M6 and Mphi 75 devices and, remotely, by the contribution of Doctor Iliya Todorov, Director of the Educational Committee for the International Federation of Manual-Musculoskeletal Medicine and President of the Bulgarian Society of Manual-Musculoskeletal Medicine.
It fell to her to focus on the use of MLS® for treating lower limb pathologies by presenting many clinical cases.
Participants’ questions to Doctor Todorov in order to better understand the therapy’s value concentrated on three points:
- how much MLS® speeds up recovery compared with standard timescales;
- the possibility of using MLS® Laser Therapy effectively during post-surgery;
- when it is advisable to begin treating a patient with a muscle or tendon injury.
“The interaction between the participants and the speaker was useful in order to answer questions in real-time, – Giacomo Granozio, ASA’s Export Area Manager explains – allowing an exchange of points of view and of experiences in the field”. The same opinion was held by Doctor Todorov who underlined how this meeting represented “a great opportunity to share knowledge and opinions with local physicians” and who hopes that “the cases presented will be useful for their daily practice”. Celine, Gaia Genomic’s CEO, is convinced of this: “The Professor’s speech had a great impact on the participants who, after her presentation, were convinced of the concrete benefits of MLS®”.