Medica 2022: ASA doubles
For the 2022 edition of Medica, ASA steps on the throttle by adding to the institutional stand - traditionally used to host business meetings - a satellite exhibition space in pavilion 4, focused on physiotherapy. An absolute novelty for the company which has aimed to highlight the transversal nature of its therapies, with a specific focus on the use of MLS® Laser Therapy for post-Covid rehabilitation.

“Once again this year Medica is confirmed to be an important appointment. The absence of some players made itself felt, but with more than 80,000 visitors, the fair remains an opportunity to meet the many foreign partners thanks to specially planned meetings at the main stand, where the protagonists were our top-of-the-range devices both for Qs Magneto-therapy and for MLS® Laser Therapy and Hilterapia®.”
explains Carlo Marchesini, ASA Marketing Manager.


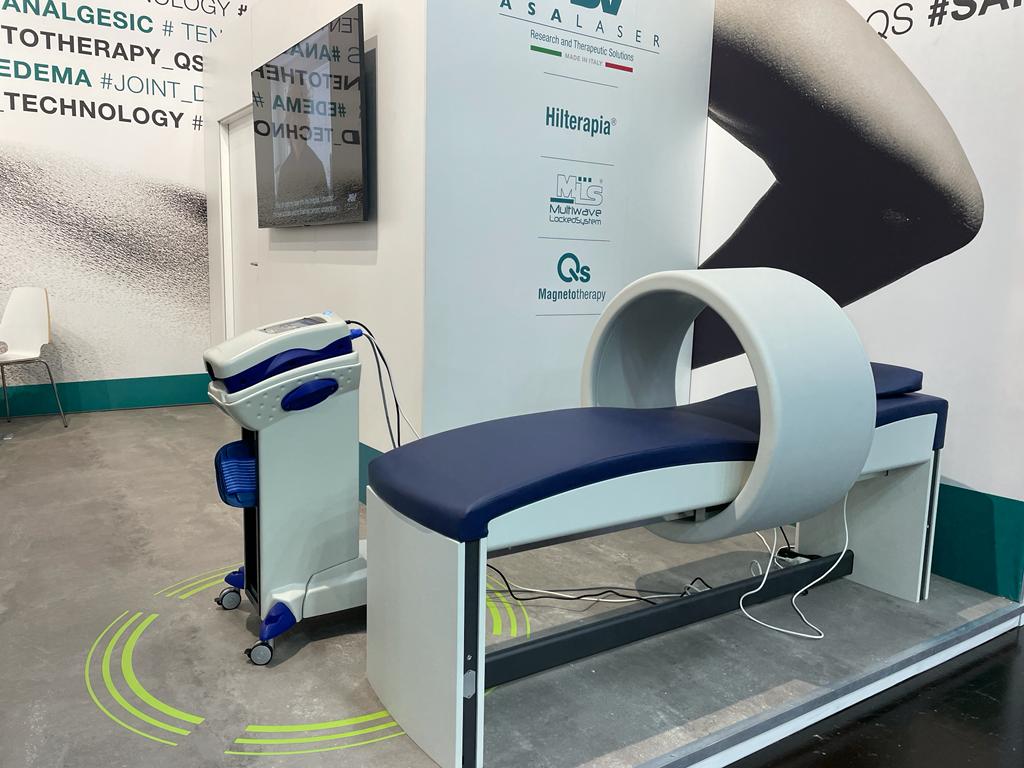
Focus therefore on MiS, HIRO TT and PMT Qs, supported by the M8 device also present in pavilion 4.
“In this second stand we displayed a kind of artistic installation featuring the only truly robotic device in the physiotherapy sector: M8. The feedback we received was very positive and our presence in this pavilion was noticed”.




“The decision to extend the company's presence to this pavilion as well", says Giacomo Granozio, ASA Area Manager, "allowed us not only to hold discussions with the sellers of other brands with whom we shared impressions of the current market, but above all to be able to define a general picture of the current technological solutions available for physical therapy and rehabilitation”.
Giulia Randazzo, Product Specialist ASA, adds:
“Pavilion 4 was a good opportunity to establish contact with dealers and at the same time with end users, inviting them to view our full range in Pavilion 11. Visitors interested in the products mainly asked for insights into the physical principle of the operation, the areas of application, the diseases which can be treated and some advice on which device to choose. The questions were very specific, a sign that awareness in the field of physical therapy is greatly increasing compared to a few years ago. Time therefore to implement our activity further”.




Precisely in the name of corporate development and of the new planning with a view to 2023, we held meetings with partners from different corners of the world, with the sole exception of Russia and Asia.
“The final balance for 2022 and the activities and budget for a successful 2023 were the crux of the meetings held during the fair, which always represents a valid opportunity to shorten the distance with our collaborators and to organise concrete actions aimed at growth" concludes Roberto Terruzzi, ASA Area Manager.
“Medica 2022 confirmed our therapeutic offer dedicated to the world of laser therapy as having a leading role, thanks also to the double presence which allowed the general public to become aware of the advantages of the only robotic laser device currently on the market. Strengthened by the results, the company looks resolutely towards 2023 during which, together with celerating its 40th anniversary, new solutions will be launched aimed at improving the well-being of patients and the activity of professionals in the medical sector”
Federico Castellani, ASA Sales Director, concludes.











