In India with MLS® between training courses and seminars
MLS® Laser Therapy and its application devices were the protagonists of a series of training courses and seminars dedicated to the Indian market promoted by ASA with the collaboration of the local distributor Biotech India.
“The initiatives we organised – explains Beniamino Caruso, ASA Area Manager – have allowed us to emphasise the valuable characteristics and benefits of MLS® Laser Therapy, providing useful information and details both to our partner Biotech, and to physiotherapy professionals of several first-class local hospitals”.
Specifically, the seminar “CPE (Continuous Physiotherapy Education) on the new frontier of high power laser therapy”, held at the new Hyderabad headquarters of the Yashoda Hospital – one of the largest facilities in the country – was attended by 90 Doctors from the same hospital and from other hospitals in the city.

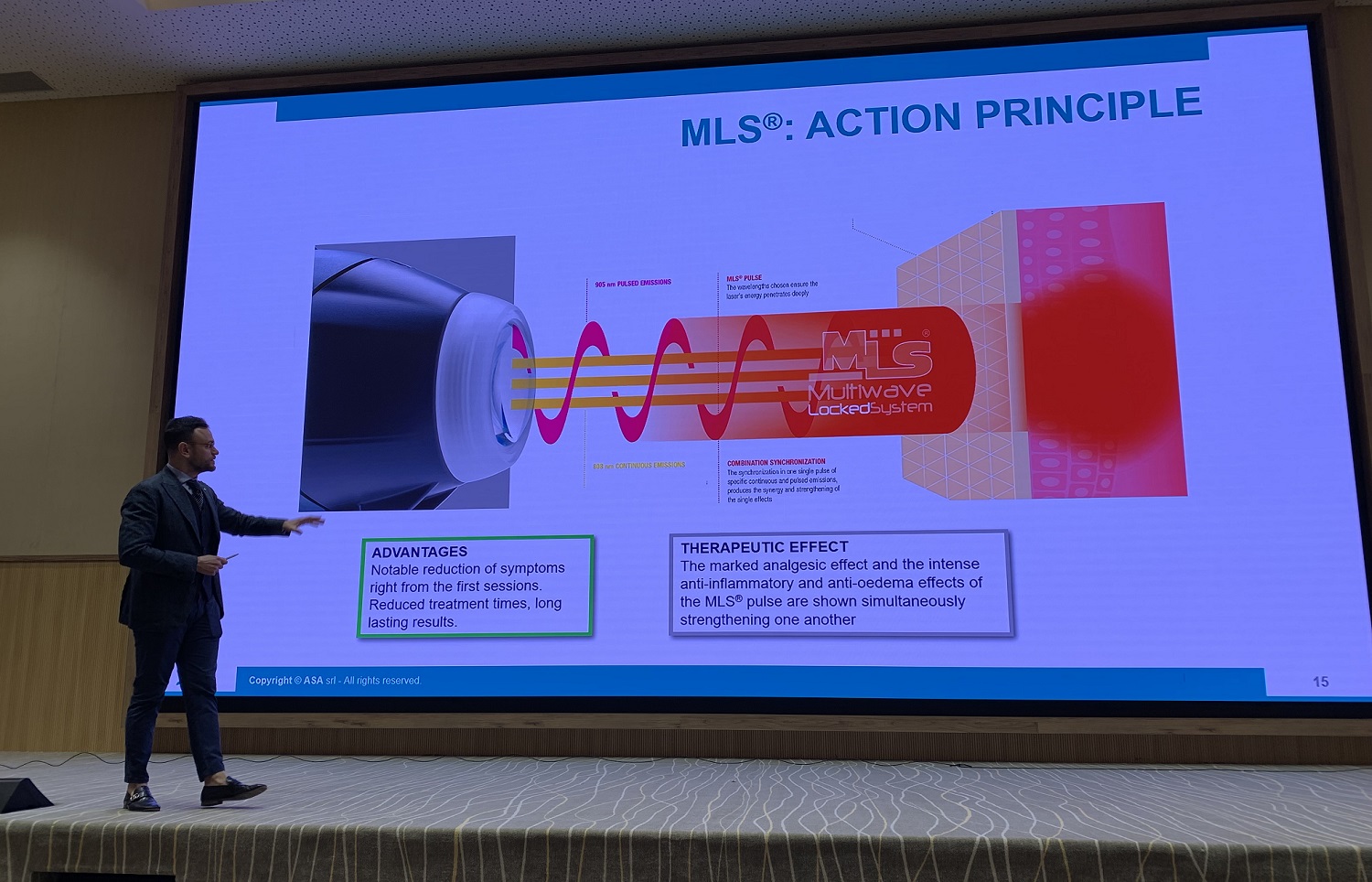


“The way the event was structured was much appreciated by those who took part in it, as it included both a short presentation of ASA and the Mphi 75 and M8 devices, physically present at the seminar, and a focus on MLS® Laser Therapy, from biological effects to application methods, accompanied by some particularly explanatory clinical cases”.
The speaker of this second part of the meeting was Salvatore Germano (ASA Field Application Specialist), who concluded his speech with a hands-on session on some volunteers. During the 4-hour meeting, in order to encourage interaction between those present and to stimulate a debate, two question-and-answer sessions were included, which allowed us to provide more in-depth information about the therapy.
MLS® was also the subject of another meeting held at the Lupin Rehabilitation and Physiotherapy Centre in Mumbai, which was recently equipped with an Mphi 75 device.
“It was a very important occasion, not only to provide useful indications on different topics such as, for example, the global approach, the meaning of the different parameters used in the device, the difference between spot application and scanning, the treatment of trigger points and how to correctly perform routine maintenance on the machine, but also to visit the facility that chose us”.
The positive outcome of the visit has been confirmed by the new opportunities that have developed: in fact, the ASA staff has been invited to repeat the event again at the Yashoda Hospital and other locations in the city of Pune.










