Slovenia: in-presence training resumes
Marked by safety and full compliance with the provisions for avoiding the spread of Covid-19, ASA resumes in-presence training, starting from Slovenia.
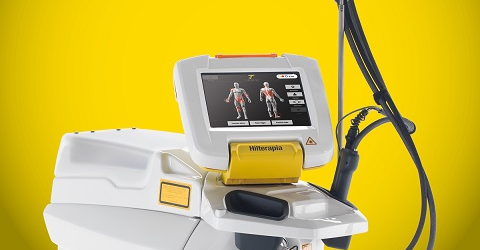
On 1st October, hosted by the local distributor Manet, company staff held a training session focusing on Hilterapia® and addressed to physiotherapists who own centres which also specialise in treating athletes.
“The meeting scheduled a theoretical part which highlighted the basics of laser therapy and the distinctive features of Hilterapia® - Roberto Terruzzi, ASA’s senior export manager ASA, explains – and a session of clinical application, followed by a discussion on a series of real-life cases. The many questions and the participants’ proactivity were the first index of the training’s success, subsequently also confirmed by a second training session carried out for The Physiotherapy Centre, part of the “Terme Ptuj” spa complex and equipped with MiS®”.


An ad hoc programme, divided into 3 sections, was devised for the facility’s physiotherapy team:
- a theoretical section on the principles of laser and the technical specifications of MiS
- a specific section dedicated to explaining the user interface and the treatment protocols
- a detailed on stage analysis
“We carried out various different treatments directly on the course’s participants so they could tangibly understand the application methods and the effects which can be obtained, The Head of the Centre was very satisfied with the meeting and underlined that it represented an important moment of discussion to promote the best use of the device”.


The Slovenia mission continued the following day at the “ARTROS reha” private clinic, where HIRO TT is their feather in the cap.
This is asserted by one of the clinic’s physiotherapists who works with this device on a daily basis, obtaining results which are not comparable with those of other manufacturers.
Precisely in order to maximise the value of the effects which can be obtained using HIRO TT, and thanks to the contribution by Salvatore Germano, ASA’s Field Application Specialist, the therapeutic work in dynamic and proprioceptive mode was analysed during the visit, providing valuable indications for improving the professional’s performance.