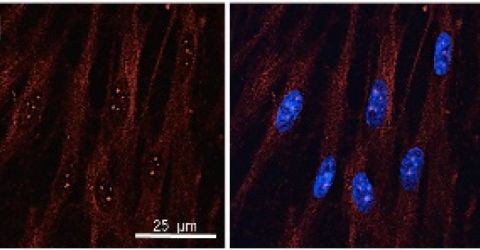
MiS and its effects when treating cerebral ischaemia
Cerebral ischaemia is a pathology caused by the reduced flow of blood to the brain and it represents the third cause of death in Europe and North America. At the moment, the only possible therapeutic approach is to quickly restore the flow of blood and the only medicinal product currently used is the tissue plasminogen activator (tPA).
However, tPA can give serious side effects, it must be administered within three hours of the ischaemic episode and is not suitable for all patients.
This is why new therapeutic approaches are being studied, with a wider application window and fewer side effects in order to improve results from a therapeutic point of view.
Laser therapy is already used in various fields: to improve wound healing, for certain respiratory diseases, etc., and it could prove to be an innovative and promising therapy for cerebral ischaemia also, thanks to its anti-inflammatory and anti-apoptotic effect.
The research: NIR Laser Photobiomodulation Induces Neuroprotection in an In Vitro Model of Cerebral Hypoxia/Ischemia
The study aims to identify a protocol capable of inducing neuroprotection and the analysis of molecular mechanisms activated by an MLS-MiS laser with dual wavelength in near-infrared (NIR) in a hypoxia/ischaemia model on a hippocampus organotypic slice culture.
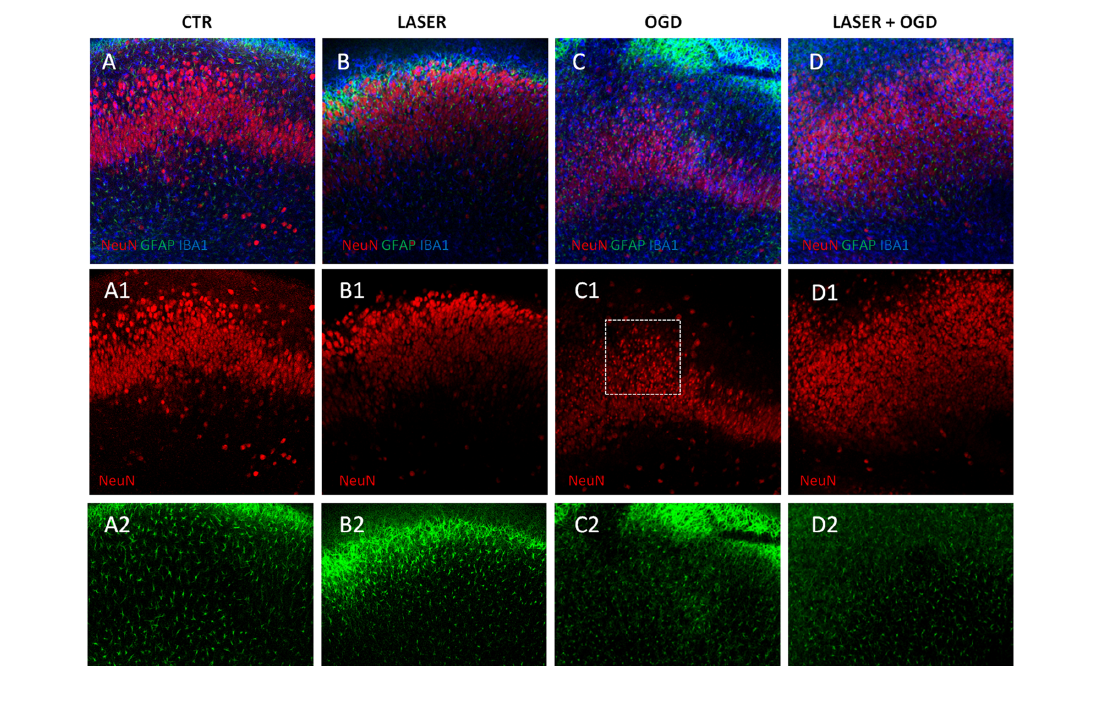
First, the hippocampus slice cultures underwent oxygen and glucose deprivation (OGD) for 30 minutes and were then treated with NIR laser immediately after the OGD, 30 minutes after and 60 minutes after, in order to establish a therapeutic window.
The results were assessed by means of confocal microscopy and the analysis of the proteins involved in the neuroinflammation and the synaptic activity and show that the changes in the morphology and in the proteins induced by the OGD are effectively contrasted by MLS-MiS laser treatment.
To conclude, the results of this study allow defining the neuroprotective mechanisms of the laser therapy carried out by MLS-MiS, to highlight its anti-inflammatory effect and to underline its potential benefit when treating cerebral ischemia.
“NIR Laser Photobiomodulation Induces Neuroprotection in an In Vitro Model of Cerebral Hypoxia/Ischemia”
E. Gerace, F. Cialdai, E. Sereni, D. Lana, D. Nosi, M.G. Giovannini, M. Monici, G. Mannaioni
Molecular Neurobiology, 2021